A pine is any conifer tree in the genus Pinus, /ˈpiːnuːs/, of the family Pinaceae. Pinus is the sole genus in the subfamily Pinoideae. The Plant List compiled by the Royal Botanic Gardens, Kew and Missouri Botanical Garden accepts 126 species names of pines as current, together with 35 unresolved species and many more synonyms.
Etymology
The modern English name pine derives from Latin pinus which some have traced to the Indo-European base *pīt- ‘resin’ (source of English pituitary). In the past (pre-19th century) they were often known as fir, from Old Norse fura, by way of Middle English firre. The Old Norse name is still used for pines in some modern north European languages, in Danish fyr, in Norwegian fura/fure/furu, Swedish fura/furu, Dutch vuren, and German Föhre, but in modern English, fir is now restricted to fir (Abies) and Douglas-fir (Pseudotsuga).
Taxonomy, nomenclature and codification
Distribution

Description

Pine trees are evergreen, coniferous resinous trees (or, rarely, shrubs) growing 3–80 m (10–260 ft) tall, with the majority of species reaching 15–45 m (50–150 ft) tall. The smallest are Siberian dwarf pine and Potosi pinyon, and the tallest is a 81.79 m (268.35 ft) tall ponderosa pine located in southern Oregon's Rogue River-Siskiyou National Forest.
Foliage
Cones
Pines are mostly monoecious, having the male and female cones on the same tree, though a few species are sub-dioecious with individuals predominantly, but not wholly, single-sex. The male cones are small, typically 1–5 cm long, and only present for a short period (usually in spring, though autumn in a few pines), falling as soon as they have shed their pollen. The female cones take 1.5–3 years (depending on species) to mature after pollination, with actual fertilization delayed one year. At maturity the female cones are 3–60 cm long. Each cone has numerous spirally arranged scales, with two seeds on each fertile scale; the scales at the base and tip of the cone are small and sterile, without seeds. The seeds are mostly small and winged, and are anemophilous (wind-dispersed), but some are larger and have only a vestigial wing, and are bird-dispersed (see below). At maturity, the cones usually open to release the seeds, but in some of the bird-dispersed species (e.g. whitebark pine), the seeds are only released by the bird breaking the cones open. In others, the seeds are stored in closed ("serotinous") cones for many years until an environmental cue triggers the cones to open, releasing the seeds. The most common form of serotiny is pyriscence, in which a resin binds the cones shut until melted by a forest fire.
Ecology


Use

Pines are among the most commercially important tree species valued for their timber and wood pulp throughout the world. In temperate and tropical regions, they are fast-growing softwoods that will grow in relatively dense stands, their acidic decaying needles inhibiting the sprouting of competing hardwoods. Commercial pines are grown in plantations for timber that is denser, more resinous, and therefore more durable than spruce (Picea). Pine wood is widely used in high-value carpentry items such as furniture, window frames, panelling, floors and roofing, and the resin of some species is an important source of turpentine.

References
Bibliography
Etymology
The modern English name pine derives from Latin pinus which some have traced to the Indo-European base *pīt- ‘resin’ (source of English pituitary). In the past (pre-19th century) they were often known as fir, from Old Norse fura, by way of Middle English firre. The Old Norse name is still used for pines in some modern north European languages, in Danish fyr, in Norwegian fura/fure/furu, Swedish fura/furu, Dutch vuren, and German Föhre, but in modern English, fir is now restricted to fir (Abies) and Douglas-fir (Pseudotsuga).
Taxonomy, nomenclature and codification
Pines are gymnosperms. The genus is divided into three subgenera, which can be distinguished by cone, seed, and leaf characters:
- Pinus subg. Pinus, the yellow, or hard pine group, generally with harder wood and two or three needles per fascicle
- Pinus subg. Ducampopinus, the foxtail or pinyon group
- Pinus subg. Strobus, the white, or soft pine group, generally with softer wood and five needles per fascicle

A Khasi pin in Benguet, Philippines.
Most regions of the Northern Hemisphere (see List of pines by region) host some native species of pines. One species (Sumatran pine) crosses the equator in Sumatra to 2°S. In North America, various species occur in regions at latitudes from as far north as 66°N to as far south as 12°N.
Various species have been introduced to temperate and subtropical regions of both hemispheres, where they are grown as timber or cultivated as ornamental plants in parks and gardens. A number of such introduced species have become invasive and threaten native ecosystems.
Huangshan pine (Pinus hwangshanensis), Anhui, China.
Description

Ancient Pinus longaeva, Nevada, USA
Pine trees are evergreen, coniferous resinous trees (or, rarely, shrubs) growing 3–80 m (10–260 ft) tall, with the majority of species reaching 15–45 m (50–150 ft) tall. The smallest are Siberian dwarf pine and Potosi pinyon, and the tallest is a 81.79 m (268.35 ft) tall ponderosa pine located in southern Oregon's Rogue River-Siskiyou National Forest.
The bark of most pines is thick and scaly, but some species have thin, flaky bark. The branches are produced in regular "pseudo whorls", actually a very tight spiral but appearing like a ring of branches arising from the same point. Many pines are uninodal, producing just one such whorl of branches each year, from buds at the tip of the year's new shoot, but others are multinodal, producing two or more whorls of branches per year. The spiral growth of branches, needles, and cone scales are arranged in Fibonacci number ratios. The new spring shoots are sometimes called "candles"; they are covered in brown or whitish bud scales and point upward at first, then later turn green and spread outward. These "candles" offer foresters a means to evaluate fertility of the soil and vigour of the trees.
Pines are long-lived, typically reaching ages of 100–1,000 years, some even more. The longest-lived is the Great Basin bristlecone pine, Pinus longaeva. One individual of this species, dubbed "Methuselah", is one of the world's oldest living organisms at around 4,600 years old. This tree can be found in the White Mountains of California. An older tree, now cut down, was dated at 4,900 years old. It was discovered in a grove beneath Wheeler Peak and it is now known as "Prometheus" after the Greek immortal.
Bark chips.
Foliage

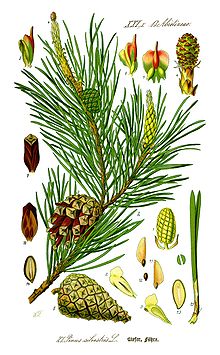
Illustration of needles, cones, and seeds of Scots pine (Pinus sylvestris).
Pines have four types of leaf :
- Seed leaves (cotyledons) on seedlings, born in a whorl of 4–24.
- Juvenile leaves, which follow immediately on seedlings and young plants, 2–6 cm long, single, green or often blue-green, and arranged spirally on the shoot. These are produced for six months to five years, rarely longer.
- Scale leaves, similar to bud scales, small, brown and non-photosynthetic, and arranged spirally like the juvenile leaves.
- Needles, the adult leaves, which are green (photosynthetic), bundled in clusters (fascicles) of 1–6, commonly 2–5, needles together, each fascicle produced from a small bud on a dwarf shoot in the axil of a scale leaf. These bud scales often remain on the fascicle as a basal sheath. The needles persist for 1.5–40 years, depending on species. If a shoot is damaged (e.g. eaten by an animal), the needle fascicles just below the damage will generate a bud which can then replace the lost leaves.
Pines are mostly monoecious, having the male and female cones on the same tree, though a few species are sub-dioecious with individuals predominantly, but not wholly, single-sex. The male cones are small, typically 1–5 cm long, and only present for a short period (usually in spring, though autumn in a few pines), falling as soon as they have shed their pollen. The female cones take 1.5–3 years (depending on species) to mature after pollination, with actual fertilization delayed one year. At maturity the female cones are 3–60 cm long. Each cone has numerous spirally arranged scales, with two seeds on each fertile scale; the scales at the base and tip of the cone are small and sterile, without seeds. The seeds are mostly small and winged, and are anemophilous (wind-dispersed), but some are larger and have only a vestigial wing, and are bird-dispersed (see below). At maturity, the cones usually open to release the seeds, but in some of the bird-dispersed species (e.g. whitebark pine), the seeds are only released by the bird breaking the cones open. In others, the seeds are stored in closed ("serotinous") cones for many years until an environmental cue triggers the cones to open, releasing the seeds. The most common form of serotiny is pyriscence, in which a resin binds the cones shut until melted by a forest fire.
Ecology

A prescribed fire in a European black pine (Pinus nigra) woodland, Portugal.
Pines grow well in acid soils, some also on calcareous soils; most require good soil drainage, preferring sandy soils, but a few (e.g. lodgepole pine), will tolerate poorly drained wet soils. A few are able to sprout after forest fires (e.g. Canary Island pine). Some species of pines (e.g. bishop pine), need fire to regenerate, and their populations slowly decline under fire suppression regimes. Several species are adapted to extreme conditions imposed by elevation and latitude (e.g. Siberian dwarf pine, mountain pine, whitebark pine and the bristlecone pines). The pinyon pines and a number of others, notably Turkish pine and gray pine, are particularly well adapted to growth in hot, dry semi-desert climates.
The seeds are commonly eaten by birds, such as grouse, crossbills, jays, nuthatches, siskins, woodpeckers, and squirrels. Some birds, notably the spotted nutcracker, Clark's nutcracker and Pinyon jay, are of importance in distributing pine seeds to new areas. Pine needles are sometimes eaten by some Lepidoptera (butterfly and moth) species (see list of Lepidoptera that feed on pines), the Symphytan species pine sawfly, and goats.

Logging Pinus ponderosa, Arizona, USA.
Use

Pine cone.
Pines are among the most commercially important tree species valued for their timber and wood pulp throughout the world. In temperate and tropical regions, they are fast-growing softwoods that will grow in relatively dense stands, their acidic decaying needles inhibiting the sprouting of competing hardwoods. Commercial pines are grown in plantations for timber that is denser, more resinous, and therefore more durable than spruce (Picea). Pine wood is widely used in high-value carpentry items such as furniture, window frames, panelling, floors and roofing, and the resin of some species is an important source of turpentine.

Pinus sylvestris prepared for transport, Hungary.
Many pine species make attractive ornamental plantings for parks and larger gardens with a variety of dwarf cultivars being suitable for smaller spaces. Pines are also commercially grown and harvested for Christmas trees. Pine cones, the largest and most durable of all conifer cones, are craft favorites. Pine boughs, appreciated especially in wintertime for their pleasant smell and greenery, are popularly cut for decorations. A number of species are attacked by nematodes, causing pine wilt disease, which can kill some quickly. Pine needles are also used for making decorative articles like baskets, trays, pots, etc. This Native American skill is now being replicated across the world. Pine needle handicrafts are made in the US, Canada, Mexico, Nicaragua and India. Pine needles serve as food for various Lepidoptera. See List of Lepidoptera that feed on pines.
Tongue and groove solid pine flooring
Because pines have no insect or decay resistant qualities after logging, they are generally recommended for construction purposes as indoor use only (ex. indoor drywall framing). This wood left outside can be expected to last no more than 12–18 months depending on the local climate. It is commonly referred to by several different names which include North American timber, SPF (spruce, pine, fir) and whitewood.
Farming
When grown for sawing timber, pine plantations can be harvested after 30 years, with some stands being allowed to grow up to 50 (as the wood value increases more quickly as the trees age). Imperfect trees (such as those with bent trunks or forks, smaller trees, or diseased trees) are removed in a "thinning" operation every five to ten years. Thinning allows the best trees to grow much faster, because it prevents weaker trees from competing for sunlight, water, and nutrients. Young trees removed during thinning are used for pulpwood, while most older ones are good enough for saw timber.
The final wood quality can be improved by pruning small branches at ages 5, 7, and 9. Pruning usually goes up to a height of 6 metres (20 ft). This results in smooth timber with no knots, which is considerably more valuable.
A 30-year-old commercial pine tree grown in good conditions will be about 0.3 metres (1.0 ft) in diameter and about 20 metres (66 ft) high. After 50 years, the same tree will be about 0.5 metres (1.6 ft) in diameter and 25 metres (82 ft) high, and its wood will be worth about 7 times as much as the 30-year-old tree.
Trees are planted 3 to 4 metres apart, or about 1000 per hectare (100,000 per square kilometer).
Food
Some species have large seeds, called pine nuts, that are harvested and sold for cooking and baking. They are an essential ingredient of Pesto alla genovese.
Edible seeds of the Korean pine (Pinus koraiensis).
The soft, moist, white inner bark (cambium) found clinging to the woody outer bark is edible and very high in vitamins A and C. It can be eaten raw in slices as a snack or dried and ground up into a powder for use as an ersatz flour or thickener in stews, soups, and other foods, such as bark bread. Adirondack Indians got their name from the Mohawk Indian word atirú:taks, meaning "tree eaters".
Biomedical
A tea made by steeping young, green pine needles in boiling water (known as "tallstrunt" in Sweden) is high in vitamins A and C.
Pine has been listed as one of the 38 substances used to prepare Bach flower remedies, a kind of alternative medicine promoted for its effect on health. However, according to Cancer Research UK, "there is no scientific evidence to prove that flower remedies can control, cure or prevent any type of disease, including cancer".
Notes- ^ Sunset Western Garden Book, 1995:606–607
- ^ "The Plant List Version 1.1". Retrieved 15 December 2015.
- ^ "Where Are You From? - Credo Reference". credoreference.com.
- ^ a b Burton Verne Barnes; Warren Herbert Wagner (January 2004). Michigan Trees: A Guide to the Trees of the Great Lakes Region. University of Michigan Press. pp. 81–. ISBN 0-472-08921-8.
- ^ "Pinus ssp. (tree), General Impact". Global Invasive Species Database. Invasive Species Specialist Group. 13 March 2006. Retrieved 2 March 2011.
- ^ Fattig, Paul (2011-01-23). "Tallest of the tall" Mail Tribune (Medford, Oregon). Retrieved 2011-01-27.
- ^ Ryan, Michael; David M. Richardson (December 1999). "The Complete Pine". BioScience 49 (12): 1023–1024. doi:10.2307/1313736.
- ^ "The Pine Plantation Rotation" (PDF). Forests NSW. Retrieved April 2016.
- ^ Frank A. Roth II, Extension Forester. "Thinning to improve pine timber". (PDF). University of Arkensas Division of Agriculture. Retrieved April 2016.
- ^ D. S. Vohra (1 June 2004). Bach Flower Remedies: A Comprehensive Study. B. Jain Publishers. p. 3. ISBN 978-81-7021-271-3. Retrieved 2 September 2013.
- ^ "Flower remedies". Cancer Research UK. Retrieved September 2013.
- Farjon, A. 1984, 2nd edition 2005. Pines. E. J. Brill, Leiden. ISBN 90-04-13916-8.
- Little, E. L., Jr., and Critchfield, W. B. 1969. Subdivisions of the Genus Pinus (Pines). US Department of Agriculture Misc. Publ. 1144 (Superintendent of Documents Number: A 1.38:1144).
- Richardson, D. M. (ed.). 1998. Ecology and Biogeography of Pinus. Cambridge University Press, Cambridge. 530 p. ISBN 0-521-55176-5.
- Sulavik, Stephen B. 2007. Adirondack; Of Indians and Mountains, 1535-1838. Purple Mountain Press, Fleischmanns, NY. 244 p. ISBN 1-930098-79-0 ISBN 978-1-930098-79-4.
- Mirov, N. T. 1967. The Genus Pinus. Ronald Press, New York (out of print).
- Classification of pines
- Gymnosperm Database - Pinus
- Mirov, N. T.; Stanley, R. G. (1959). "The Pine Tree". Annual Review of Plant Physiology 10: 223. doi:10.1146/annurev.pp.10.060159.001255.
- Philips, Roger. Trees of North America and Europe, Random House, Inc., New York ISBN 0-394-50259-0 1979.,
Wikipedia












