Author
For further details log on website :
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0130672
- Published: June 23, 2015
- http://dx.doi.org/10.1371/journal.pone.0130672
Soil microbial communities undergo rapid shifts following modifications in environmental conditions. Although microbial diversity changes may alter soil functioning, the in situ temporal dynamics of microbial diversity is poorly documented. Here, we investigated the response of fungal and bacterial diversity to wheat straw input in a 12-months field experiment and explored whether this response depended on the soil management history (grassland vs. cropland). Seasonal climatic fluctuations had no effect on the diversity of soil communities. Contrastingly fungi and bacteria responded strongly to wheat regardless of the soil history. After straw incorporation, diversity decreased due to the temporary dominance of a subset of copiotrophic populations. While fungi responded as quickly as bacteria, the resilience of fungal diversity lasted much longer, indicating that the relative involvement of each community might change as decomposition progressed. Soil history did not affect the response patterns, but determined the identity of some of the populations stimulated. Most strikingly, the bacteria Burkholderia, Lysobacter and fungi Rhizopus, Fusarium were selectively stimulated. Given the ecological importance of these microbial groups as decomposers and/or plant pathogens, such regulation of the composition of microbial successions by soil history may have important consequences in terms of soil carbon turnover and crop health.
Figures
Citation: Tardy V, Chabbi A, Charrier X, de Berranger C, Reignier T, Dequiedt S, et al. (2015) Land Use History Shifts In Situ Fungal and Bacterial Successions following Wheat Straw Input into the Soil. PLoS ONE 10(6): e0130672. doi:10.1371/journal.pone.0130672
Editor: Eiko Eurya Kuramae, Netherlands Institute of Ecology (NIOO/KNAW), NETHERLANDS
Received: December 9, 2014; Accepted: May 22, 2015; Published: June 23, 2015
Copyright: © 2015 Tardy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This work was supported by the Agence Nationale de Recherche (ANR) as part of the ANR Systerra project DIMIMOS (ANR-08-STRA-06) and by a grant from the Regional Council of Burgundy. It benefited from the technical facilities of the GenoSol platform of the infrastructure ANAEE-France [ANAlysis and Experimentations on Ecosystems, ANR project ‘Investments for the Future’ (ANR-11-INBS-0001)], as well as from the ORE-ACBB (http://www.soere-acbb.com/index.php/fr/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Soils are highly complex, heterogeneous matrices which shelter a huge diversity of organisms [1,2]. With thousands of different species hosted per gram of soil, microbial communities account for a large part of this biodiversity [3,4]. As a major component of the biosphere, soil also offers a support for plant and animal development, human activities, and is directly exposed to variations in climatic conditions. It is consequently a dynamic environment, and there is a considerable body of evidence that soil microbial diversity responds strongly to changes in soil conditions [5–7].
One key example is the pattern of microbial community dynamics induced by the addition of organic compounds such as plant debris into the soil [8–14]. Many studies have indeed reported a progressive and orderly pattern of community development, as regards the concept of species succession commonly employed to explain community dynamics in above-ground terrestrial plant communities [15]. Most of the community changes occur during the first month after the input, mainly attributed to fast-growing copiotrophic populations that take advantage of the readily degradable C-compounds (i.e. soluble compounds, cellulose, hemicellulose) in the plant residues [14,16,17]. Subsequently, more specialized slow-growing populations develop on the remaining recalcitrant C-compounds (i.e. lignin) due to their greater catabolic capacities compared to the early-stimulated populations [8,11,12,18]. Such patterns of diversity dynamics have been demonstrated for bacteria [13,14] and fungi [17]. In addition, since bacteria are assumed to harbor lower capacities for decomposing recalcitrant compounds than fungi [19], it is generally accepted that bacteria dominate the initial stages of decomposition whereas fungi dominate the later stages [20–22]. However, this is mainly based on quantitative estimates of bacterial and fungal communities. In contrast, studies addressing the response of the taxonomic composition of soil bacterial and fungal communities to plant residues addition are scarce and the relative contribution of bacterial vs. fungal diversity to the different stages of plant residues decomposition remains poorly documented [22,23].
In addition, while many studies have shown that land use management [24–28] as well as plant litter quality (i.e. initial C/N ratio, lignin content, etc…) [11,14,29] are important drivers of soil microbial diversity in a given soil, it is not known if the response of soil microbial communities to the addition of a fixed quality of plant residue depends on the soil’s history. This question is, however, of major importance since the intensity of the decomposition process and the resulting ecosystem services may depend on the diversity of the microbial populations responding to the input [14,30,31].
The aim of this study was to investigate the importance of soil management history in the response of the taxonomic diversity of bacterial and fungal communities to the incorporation of wheat straw. For this, a field experiment was set up at the long-term observatory of Lusignan (France). Briefly, the site was composed of two adjacent plots, each with a particular land use history (17 years grassland vs. 20 years cropland). Each plot was subdivided into 6 sub-plots, three of which were amended with wheat residues while the other three represented unamended controls. Following wheat addition, the dynamics of microbial diversity (fungal and bacterial) was followed in each sub-plot over a one-year period by high throughput sequencing of ribosomal genes. The site was kept vegetation-free throughout the experiment to avoid an additional response of microbial communities to plant development. To our knowledge this is the first study of the fine in situ characterization of the response of bacterial and fungal diversity to seasonal climatic fluctuations and plant residues addition in relation to the soil’s land use history.
Materials and Methods
Site description and sampling
The field experiment was set up on the long-term observatory for environmental research at Lusignan, France (SOERE-ACBB, http://www.soere-acbb.com/index.php/fr/). The soil on the site is a loamy textured Cambisol and the climate is oceanic with a mean annual temperature and precipitation of 10.5°C and 800 mm, respectively [32]. The experimental site was set up in two phases. First, in April 2011, the two main plots were established (S1 Fig). They were each 5×2 m in size and very close together (separated only by a 5 m pathway), but strongly contrasted in terms of land use history. One plot was set up on a soil that had been under crop rotation (wheat, barley and maize) for 20 years with annual tillage, herbicide crop treatment and nitrogen fertilization (ammonium nitrate: 100 kg/ha/year). The other plot was set up on permanent grassland (over 20 years old), fertilized (ammonium nitrate: 150 to 300 kg/ha/year) and harvested (3 to 4 times/year) for annual forage. The vicinity of the two plots was a prerequisite to ensure that differences in physicochemical and biological properties between the two plots were due to land use history, and not to spatial heterogeneity. Each plot (grassland and cropland) was weeded manually (shoots and roots) in order to remove the effect of plants on the soil microbial communities. After weeding, each plot was divided into six 0.7×0.7 m sub-plots (S1 Fig) and the site was left for 5 months to stabilize, with manual weeding every week to remove regrowth seedlings. In September 2011 the soil from each of the 6 sub-plots in each grassland and cropland plot was excavated to a depth of 10 cm. For each plot, soils from 3 of the sub-plots were amended homogeneously by mixing the excavated soil with wheat residues (250 g per sub-plots, corresponding to 5 t dry matter ha-1). Wheat residues originated from mature wheat plants (harvested 110 days after sowing) cultivated under controlled conditions (Groupe de Recherches Appliquées en Phytotechnologie, CEA Cadarache, France). The roots were separated from the shoots which were then oven-dried at 65°C for 48 h and cut to obtain straw residues 0.5 cm in length. The C/N ratio determined for wheat was 77.7 (elementar analyser Euro EA (EUROVECTOR, Milano, Italia)). The soil from the 3 other sub-plots was also mixed, but without addition of wheat straw. After mixing, the amended and non-amended soils were returned to their respective sub-plots. In this way, the site was finally composed of two plots, each representing a particular land use history (grassland vs. cropland), with two treatments per plot represented by triplicate wheat straw-amended sub-plots and triplicate non-amended control sub-plots.
To avoid horizontal transfers of organic matter between control and amended sub-plots, a protective tarp was buried to 30 cm depth around the control sub-plots. In addition, polyethylene safety nets with 10×10-mm diamond mesh were placed on the wheat straw-amended sub-plots to avoid migration of surface applied residues via runoff. Soil sampling was carried out regularly from September 2011 to September 2012, with more frequent sampling during the first month following wheat straw incorporation. This represented a total of 10 sampling dates: T0 (date of wheat straw addition); T3; T7; T22; T51; T85; T125; T211; T254; and T365 days, corresponding to 120 soil samples (3 replicates×2 treatments×2 plots×10 dates). After sampling, the soil was sieved to 2 mm, lyophilized and stored at -40°C before DNA extraction. In addition, part of the soil sampled at T0 was air dried for analysis of particle size distribution, pH, Soil Organic Carbon (SOC), Total Nitrogen (N-tot), soil C/N ratio, Soil Organic Matter (SOM), Phosphorous and Cation Exchange Capacity (CEC). Soil physicochemical properties were determined by the Soil Analysis Laboratory of INRA (ARRAS, France, http://www.lille.inra.fr/las).
DNA extraction and molecular microbial biomass determination
Microbial DNA was extracted from 1 g of all 120 soil samples, using a single procedure standardized by the GenoSol platform (http://www.dijon.inra.fr/plateforme_genosol) [33]. DNA concentrations of crude extracts were determined by electrophoresis in 1% agarose gel using a calf thymus DNA standard curve, and used as estimates of microbial molecular biomass [34]. After quantification, DNA was purified using a MinElute gel extraction kit (Qiagen, Courtaboeuf, France).
Pyrosequencing of 16S and 18S rRNA gene sequences
Bacterial and fungal diversities were determined at each sampling date, for each triplicate treatment (amended and control) of each land use history (grassland and cropland) by 454 pyrosequencing of ribosomal genes. For bacteria, a 16S rRNA gene fragment with sequence variability and the appropriate size (about 450 bases) for 454 pyrosequencing was amplified by PCR using primers F479 and R888. For fungi, an 18S rRNA gene fragment of about 350 bases was amplified using primers FR1 and FF390. Primers and PCR conditions were as described previously [7]. The PCR products were purified using a MinElute gel extraction kit (Qiagen, Courtaboeuf, France) and quantified using the PicoGreen staining Kit (Molecular Probes, Paris, France). A second PCR of 9 cycles was then conducted under similar PCR conditions with purified PCR products and ten base pair multiplex identifiers added to the primers at 5’ position to specifically identify each sample and avoid PCR bias. Finally, the PCR products were purified and quantified as previously described [7]. Pyrosequencing was then carried out on a GS FLX Titanium (Roche 454 Sequencing System).
Bioinformatics analysis of 16S and 18S rRNA gene sequences
Bioinformatics analyses were done using the GnS-PIPE developed by the GenoSol platform (INRA, Dijon, France) and initially described by Terrat et al., [33]. First, all reads were sorted according to the chosen identifiers sequences. The raw reads were then filtered and deleted based on their: (a) length, (b) number of ambiguities (Ns), and (c) primer(s) sequence(s). PERL programs were then applied to obtain strict dereplication, alignment of reads using infernal alignments [35] and clustered at 95% sequence similarity into operational taxonomic units (OTU) that cluster rare reads with abundant ones, and do not count differences in homopolymer lengths. Another homemade filtering step was then applied to eliminate potential sources of errors (e.g., PCR chimeras, sequencing errors, OTU overestimation). In order to efficiently compare the datasets and avoid biased community comparisons, the samples reads were reduced by random selection closed to the lowest datasets (4,000 and 8,000 reads for 16S- and 18S-rRNA gene sequences respectively). The retained high-quality reads were used for: (i) taxonomy-independent analyses, to determine diversity indices using the defined OTU composition, and (ii) taxonomy-based analysis using similarity approaches against dedicated reference databases from SILVA (R114 for 16S, and R111 for 18S). The raw data sets are available on the EBI database system under project accession number [PRJEB6118].
Statistical analysis
Differences in microbial communities structure between control and wheat-incorporated plots within a given land use history were characterized using UniFrac distances [36]. Non Metric Multidimensional Scaling (NMDS) was used to graphically depict differences between microbial communities. Analysis of SIMilarity (ANOSIM, 999 permutations) was used to test the significance of the observed clustering of samples on the ordination plot according to soil history. The difference in change of the microbial communities in response to wheat straw between the two land use histories was interpreted as the magnitude of the UniFrac distance between the control and the amended plots for each sampling date and was calculated as follows: magnitude of modifications for each sampling date and land use history = [Average of Inter UniFrac distances (control plots/ soil-incorporated plots)]–[((Average of Intra UniFrac distances control plots) + (Average of Intra UniFrac distances soil-incorporated plots))/2].
Heat maps were built up by applying the heatmap.2 function implemented in the gplots R package to the relative abundance values of the most dominant bacterial and fungal phyla across the samples (relative abundance > 1%). For the analysis, the datasets (soil histories) were analysed together, so the mean values of Z scores are from the total dataset. Each row was scaled so that the mean of each taxonomic group across sample types was calculated and colored according to the corresponding Z score. All these analyses were performed with the R free software (http://www.r-project.org/).
Physicochemical parameters, diversity metrics, Unifrac distance and relative abundance were compared between treatments and land use histories by two-way ANOVA and the differences between them by Fisher test (P<0.05). All these statistical calculations were carried out using XLSTAT software (Addinsoft).
Results
Soil physicochemical characteristics
Soil texture corresponded to a loamy sand soil (Table 1) and did not differ between the grassland and cropland plots. In contrast, soil chemical properties were slightly but significantly modified by land use history with higher values of SOM, SOC, N-Tot, Soil C/N and CEC in grassland soil, whereas pH and phosphorous content were higher in the cropland soil. No significant difference was observed between cropland and grassland soil for soil microbial biomass (Table 1).
Table 1. Soil physicochemical characteristics and soil microbial molecular biomass according to land use history at T0.
Impact of land use history and seasonal climatic variations on soil microbial diversity
Pyrosequencing yielded a total of 1,844,885 and 2,020,216 sequences of 16S- and 18S-rDNA with 4000 and 8000 quality sequences per sample, respectively. Diversity indices calculated from the sequence datasets obtained from control plots (without wheat addition) showed that, overall, both bacterial and fungal richnesses were higher in cropland plots than in grassland plots whatever the sampling dates (Fig 1). Contrastingly, bacterial evenness was similar in the two soils whereas fungal evenness was lower in grassland than in cropland soil. Interestingly, bacterial and fungal diversity indices did not vary significantly during the 12-month experiment whatever the soil history.
Fig 1. Dynamics of bacterial (A and C) and fungal (B and D) richness and evenness during the 12-month experiment in control (full line) and wheat-amended plots (dashed line) for each soil history: grassland (grey) and cropland (black).
Error bars denote standard deviation of biological replicates (n = 3). Symbols in superscript indicate significant differences according to Fisher test (P<0.05) between control and corresponding wheat amended sub-plots for each grassland (stars) and cropland (circles) history.
NMDS analysis of the full bacterial- (Fig 2A) and fungal-sequences datasets (Fig 2B) highlighted distinct microbial community structure between the control subplots depending on the land use history (confirmed by Pairwise ANOSIM test (R = 0.805, P = 0.001 for bacteria and R = 0.916, P = 0.001 for fungi)). A difference between the two soils was also observed at the OTU level, with only 21.4% of bacterial and fungal OTU being shared at T0 (data not shown). The taxonomic affiliation of 16S- and 18S-rDNA sequences at T0 showed that overall the microbial phyla distribution within these two soils was dominated by Proteobacteria, followed by Actinobacteria, Firmicutes, Acidobacteria, Bacteroidetes, and Chloroflexi for bacteria, and by Basidiomycota and Ascomycota for fungi. However, the difference between the two soils was mainly due to an increase in the relative proportions of Proteobacteria (58.6 to 54.7%), Actinobacteria (11.8 to 8.5%), Acidobacteria (8.9 to 7.8%), and Basidiomycota (45.3 to 21.2%) in grassland, as compared to cropland soil which harbored higher relative proportions of Gemmatimonadetes (2.4 to 1.2%), Chloroflexi (4.5 to 1.7%), Nitrospirae (1.3 to 0.6%), and Ascomycota (55.7% to 29%).
Fig 2. Non-metric multi-dimensional scaling (NMDS) ordination plot derived from weighted pairwise Unifrac distances for (A) bacterial and (B) fungal communities over time according to soil management history: cropland (squares) and grassland (circles).
White labels represent the control treatment and grey labels represent the wheat-amended treatment. Numbers represent sampling dates and lines correspond to the three replicate samples obtained at each sampling date. Stress values for the two ordination plots were < 0.2 which indicates that these data were well-represented by the two dimensional representation. inset represents magnitude changes in Unifrac distance between the control and amended sub-plots over the year.
As observed for the diversity indices, bacterial and fungal structures (Fig 2) in the controls did not change significantly during the experiment, with all sampling dates being grouped together on the factorial map. This stability of the microbial communities was also observed when the bacterial taxonomic composition was analysed at both phylum (Fig 3) and genus levels (S2 and S3 Figs).
Fig 3. Heat map comparison of bacterial and fungal phyla detected in soils according to land management history (cropland and grassland) and treatments (control or amended) over time.
In the amended treatment, the white dashed line represents the time of wheat straw input. The captions show the Z-scores (relative abundances are expressed as median centered Z-scores between all samples, and the colors are scaled to the standard deviations. For each sampling date, the average relative abundance was obtained from the biological replicates (n = 3).
Response of soil microbial diversity to wheat residues according to land use history
Wheat residues addition led to strong modifications of soil microbial diversity whatever the soil history (Fig 1). All microbial diversity indices (bacterial and fungal) were decreased during the first week following straw incorporation (Fig 1), except for bacterial richness, which was similar in both control and amended treatments irrespective of soil history (Fig 1A). Following this first phase of decline, the indices then increased and were resilient, stabilizing at the level of the respective controls. However, resilience occurred more rapidly for bacteria (22 days for bacterial evenness) than for fungi (85 days for both fungal richness and evenness). In addition, the overall amplitude of the modifications in diversity indices was greater for fungi than for bacteria.
Microbial community structure also responded strongly to wheat addition (Fig 2). Similar patterns of community dynamics were observed for bacteria and fungi whatever the soil history, characterized by a highly dynamic phase during the first week after wheat addition followed by a slowdown and resilience of community modification. The community changes peaked after 3 days for both bacteria and fungi whatever the soil history (Fig 2), as observed for the diversity indices. However, the amplitude of the structural modifications was greater for fungi than for bacteria, and resilience of the community structure occurred more rapidly for bacteria (51 days) than for fungi (365 days), although the time of occurrence did not depend on the land use history (Fig 2).
Despite the similar community response patterns (Figs 1 and 2), the community structures (bacterial and fungal) at each sampling date could still be clearly discriminated between the two soils (grassland vs. cropland), indicating that different communities were stimulated by wheat residues (Fig 2). This observation was confirmed by the taxonomic compositions at the phyla and genera levels (Fig 3; S2 and S3 Figs). Some microbial phyla responded similarly to wheat addition (Fig 3) in the two soils. Thus, Proteobacteria and the group of sequences “unclassified” to fungal phyla were both strongly increased during the early stage following residues input. Other bacterial phyla, such as Nitrospira, Verrumicrobia, Acidobacteria, Actinobacteria, and Planctomycetes were decreased, as well as the fungal phyla Basidiomycota, Chytridiomycota, and Glomeromycota. In addition to these “shared response” groups, some phyla responded specifically according to the soil history. For example, the early increase of Fibrobacteres (from 3 to 125 days following wheat addition) occurred only in the cropland soil whereas Synergistetes and Bacteroidetes increased much later (from 85 to 254 days following wheat addition) and only in grassland soil. In the same way, Ascomycota increased mainly in the grassland soil throughout the experiment. Analysis of taxonomic composition at the genera level provided complementary information to the phyla data. For instance, the general increase in Proteobacteria was mainly explained by the stimulation of Pseudomonas and Massilia, whereas Lysobacter only increased in the cropland soil, and Burkholderia only increased in grassland soil (Fig 4). Similarly, the group of sequences “unclassified” to fungal phyla, which represented the principal group of stimulated fungal sequences regardless of soil history, mainly consisted of populations affiliated to Mucor, for which the relative abundance increased throughout the experiment; Mortierella, which also increased in both soils, but just from day 0 to day 3; and Rhizopus, which increased strongly in cropped soil but not in grassland soil (Fig 5).
Fig 4. Dynamics of the relative abundance of some bacterial genera over time, according to soil management history (cropland and grassland) and treatment: control (dark grey) and wheat-amended (light grey).
Error bars denote standard deviation of biological replicates (n = 3). Asterisks indicate significant difference between control and amended treatments at each sampling date, according to Fisher test (P<0.05).
Fig 5. Dynamics of the relative abundance of some fungal genera over time, according to soil management history (cropland and grassland) and treatment: control (dark grey) and wheat-amended (light grey).
Error bars denote standard deviation of biological replicates (n = 3). Asterisks indicate a significant difference between control and amended sub-plots at different sampling dates, according to Fisher test (P<0.05).
Discussion
Bacterial and fungal diversity and composition differed according to the soil management history (grassland vs. cropland), as reported in previous studies [26,37]. In addition, discrimination between the two soils remained stable throughout the one-year experiment, highlighting the long-lasting nature of the changes induced by past land use [26,38,39]. Since the site was kept weed-free, it is likely that the differences in soil properties accounted for a large part of the microbial communities discrimination between the two soils [40–43]. While soil texture was similar, as expected because of the vicinity of the two plots, soil chemical properties differed between the grassland and cropland soils. Thus SOC and soil nitrogen content were higher, and pH lower in the grassland soil, in agreement with other studies [39,44]. Accordingly, microbial groups previously described as mainly copiotrophic, such as Proteobacteria [14,18], or acidophilic, such as Acidobacteria [45,46]were more represented in the grassland soil than in the cropland soil where oligotrophic groups such as Gemmatimonadetes were more represented [16,47]. In addition, Ascomycota, one of the most dominant fungal groups in cultivated soils [48,49], dominated the fungal community in the cropland soil. Contrastingly, the abundance of Basidiomycota and Actinobacteria, both described as k-strategists, being favored in more stable systems such as forest [50] and natural grassland [24,51,52], was increased in the grassland soil.
In the controls, neither bacterial nor fungal diversity (i.e. richness and evenness) nor composition varied significantly during the one-year period despite the climatic variations that occurred at the experimental site (S4 Fig). This contrasted with Lauber and colleagues [6] who evidenced a significant in situ temporal variability of bacterial communities over a 7-month period. These authors showed however that a large proportion of the seasonal variations in bacterial diversity could be explained by the diversity and dynamics of plant cover and the subsequent regulation of C-inputs (i.e. root exudates and plant litter). Thus, the observed stability of microbial diversity in our study might be explained by the exclusion of plants. Interestingly, this suggests that under a buffered oceanic climate, as is the case at our experimental site, the dynamics of plant cover rather than climatic variations may shift microbial diversity during the seasons.
Soil microbial diversity responded strongly to wheat straw incorporation, in agreement with many other studies focused on the impact of plant litter on soil microbial communities [8,9,12,13,17]. Microbial richness and evenness indices showed a transient decrease following amendment, due to the early stimulation of a subset of fast growing bacterial and fungal populations developing on the most easily decomposable straw-compounds [14,17,22]. This was in agreement with the patterns of community structure, that were characterized by an initial early and highly dynamic phase followed by a second phase of slower variations leading to a resilience and stability of both bacterial and fungal communities structure. It is noteworthy that for a given community (i.e. bacteria or fungi), in spite of initial differences in terms of diversity and composition, neither the variations in diversity indices nor the patterns of community structure differed according to the soil management history. However, the kinetics and amplitude of community response did differ between bacteria and fungi. Surprisingly, the peak in communities’ modification, for both bacteria and fungi, occurred three days after the input, indicating that fungi may be as competitive as bacteria for fast development on freshly incorporated wheat straw. This is not in agreement with the commonly accepted idea that differences in physiology and metabolic capacities between bacteria and fungi may confer an advantage to bacteria over fungi during the early stages of decomposition [19,21,53]. However, recent studies using 13C labelling of plants also showed that decomposer fungi may contribute significantly to the decomposition of simple root exudates [54,55]. In addition, the quality of the wheat straw, characterized by a high C/N (77.7), may also have contributed to the concomitant, early stimulation of bacterial and fungal populations, as well as the greater amplitude of the diversity modification observed for fungi compared to bacteria. Indeed, fungi are known to be better equipped than bacteria for decomposing recalcitrant organic C-compounds [20–22] and the decomposability of the litter entering the soil is likely to be of major importance in determining the sequential stimulation of bacteria and fungi during the early phase of decomposition. In other respects, the better enzymatic abilities of fungal populations may explain why fungal communities responded up to 122 days whereas modifications of the bacterial communities occurred only during the first month (22 days) after wheat addition. This slower resilience of fungal diversity, as compared to that of bacteria, suggests that fungi may dominate the latter stages of wheat straw decomposition [19,21].
While the patterns of bacterial and fungal diversity indices and communities structure were similar, regardless of soil history, some of the populations entering the successions differed. Several bacterial phyla, such as Nitrospira, Verrumicrobia, Acidobacteria and Planctomycetes, were decreased by wheat straw incorporation, which was in agreement with other studies showing that these phyla are probably mainly composed of oligotrophic slow-growing populations [14,16,18]. In addition, Nitrospira underrepresentation might also be response of reduction of nitrogen availability due to high C/N material input. Contrastingly, Bacteroideteswere stimulated by wheat straw, but in the latter stages of wheat decomposition (i.e. 5 months after wheat addition), in agreement with previous results [29]. The Proteobacteria phylum was increased rapidly regardless of the soil history, as reported previously [29,56]. This global increase was mainly due to the stimulation of two genera occurring in both soils: Pseudomonasand Massilia that had previously been described as mainly copiotrophic bacteria being stimulated by inputs of wheat straw or more soluble C-compounds [16,57,58]. Interestingly, in addition to these genera, Burkholderia was enhanced only in the soil with a grassland history whereas Lysobacter was enhanced only in the soil with a cropland history. Recent studies also reported stimulation of Burkholderia species by plant residue inputs into the soil [23], probably because of the strong catabolic versatility of these species which enables them to degrade a wide range of C-compounds including cellulose [59]. Since this genus is also known to be closely associated with plants in the rhizosphere [23], it is likely that the long grassland history, characterized by permanent plant cover with a very dense root network, may have led to the selection of a particular diversity of Burkholderia species, compared to the adjacent cropland, hence explaining the observed discrepancy between the responses of the two soils. However, differential stimulation of this genus may be of significance for subsequent crops since many Burkholderia species are associated with plants in positive (i.e. growth promoting species) or negative (i.e. pathogens) interactions [59]. Similarly to Burkholderia, Lysobacter is known to possess strong lytic abilities to degrade various biomacromolecules [60]. However, this genushas also been shown to decrease with decreasing soil pH [60], which may explain why stimulation was observed in the cropland soil (pH 6.6), but not in the grassland soil (pH 6.0). Altogether, these results suggest that the modulation in diversity of the populations responding to plant litter input, according to the past history of the soil, may be determined either by the actual soil properties or by the diversity of the decomposers resulting from past interactions with plant cover.
Regarding the fungal communities, the observed initial decrease of the diversity indices was due to the massive increase of Mortierella, which represented up to 20 and 30% of the sequences identified at T3 in the cropland and grassland soils, respectively. This strong stimulation, observed only three days after wheat straw incorporation, suggests that this genusmay be as competitive as bacteria in decomposing the most decomposable straw C-compounds. This is in agreement with other reports that Mortierella are fast growing fungi developing on substrates rich in simple carbohydrates [61,62]. In addition, their ability to produce chitinolytic enzymes may make them successful competitors among other fungi [61], which could explain their massive and exclusive development observed at T3. Nevertheless, this competitive advantage may be limited to decomposition of the most soluble wheat compounds since Mortierella decreased from T7 and was replaced by a diversity of other newly stimulated fungi. As for bacteria, some of these new populations were shared or specifically stimulated depending on the soil history. For example, Mucor and Chaetomium were stimulated in both soils from T7 and throughout the year of experimentation, in agreement with their known contribution to plant debris decomposition in soil due to their cellulolytic, hemicellulolytic and lignolytic abilities [63,64]. The genus Sarocladium was also stimulated in both soils, but only transiently, with a peak observed at T51. Interestingly, two fungal genera responded specifically depending on the soil history. Most strikingly, Rhizopus was very weakly represented in the controls of both soils, but responded strongly and lastingly to wheat straw incorporation in the cropland soil (representing up to 15% of the sequences identified) but not in the grassland soil. This genus is a common soil mold which develops on decomposing plant litter in soil [62]. However, we cannot explain why it only responded in the cropland soil. It is possible that this selective stimulation could be of significance for the health of subsequent crops since some Rhizopus species are described as plant pathogens [61]. In contrast to Rhizopus, Fusariumwas one of the dominant fungi in both soils and was strongly stimulated in the grassland soil but not in the cropland soil. This genus is known be saprophytic, widely distributed in soil and often associated with plant debris [65]. A similar stimulation of Fusarium populations following crop residues incorporation, and the strong influence of the residues composition on the structure of the Fusarium community, has already been reported [66]. Here, we show that the stimulation of Fusarium by plant residues may also depend on the past use of the soil. In addition to potential repercussions in terms of carbon mineralization, this differential stimulation between the two soils may have consequences for subsequent crops since some of the Fusarium species are strongly associated with several diseases that cause crop yield losses and mycotoxin contamination of grain [65]. However, since all Fusarium species are not pathogenic, additional determination of Fusarium diversity at the species level would now be needed to determine whether populations differentially stimulated were pathogenic or not.
This study provides new insights into the in situ dynamics of the diversity of bacterial and fungal communities in soil. It shows that seasonal fluctuations of climatic conditions may have little impact on the diversity of these communities. While long-term differences in land use management impacted soil microbial diversity, they did not affect the patterns of community response to wheat straw incorporation in terms of diversity indices and community structure variations. Fungal diversity responded as quickly as bacterial diversity but the resilience of fungal communities lasted longer, which would indicate that whereas bacteria and fungi may both play a major role during the initial stages, fungi may dominate the latter stages of wheat decomposition. Despite similar response patterns, the identity of some of the bacterial and fungal populations in the successions was dependent on soil history. Among the specifically stimulated populations were bacterial and fungal populations of particular ecological importance, such as Burkholderia or Fusarium, both recognized for their involvement in the decomposition of organic matter, but also for their impact on crops health. This effect of soil history on population successions during the decomposition of a given quality of plant litter had not been evidenced previously. It is however of major importance since it highlights that the diversity of microbial decomposers may be driven by soil management history, with potential consequences in terms of plant residues decomposition, a transformation of significance for the C-balance in the soil environment.
Supporting Information
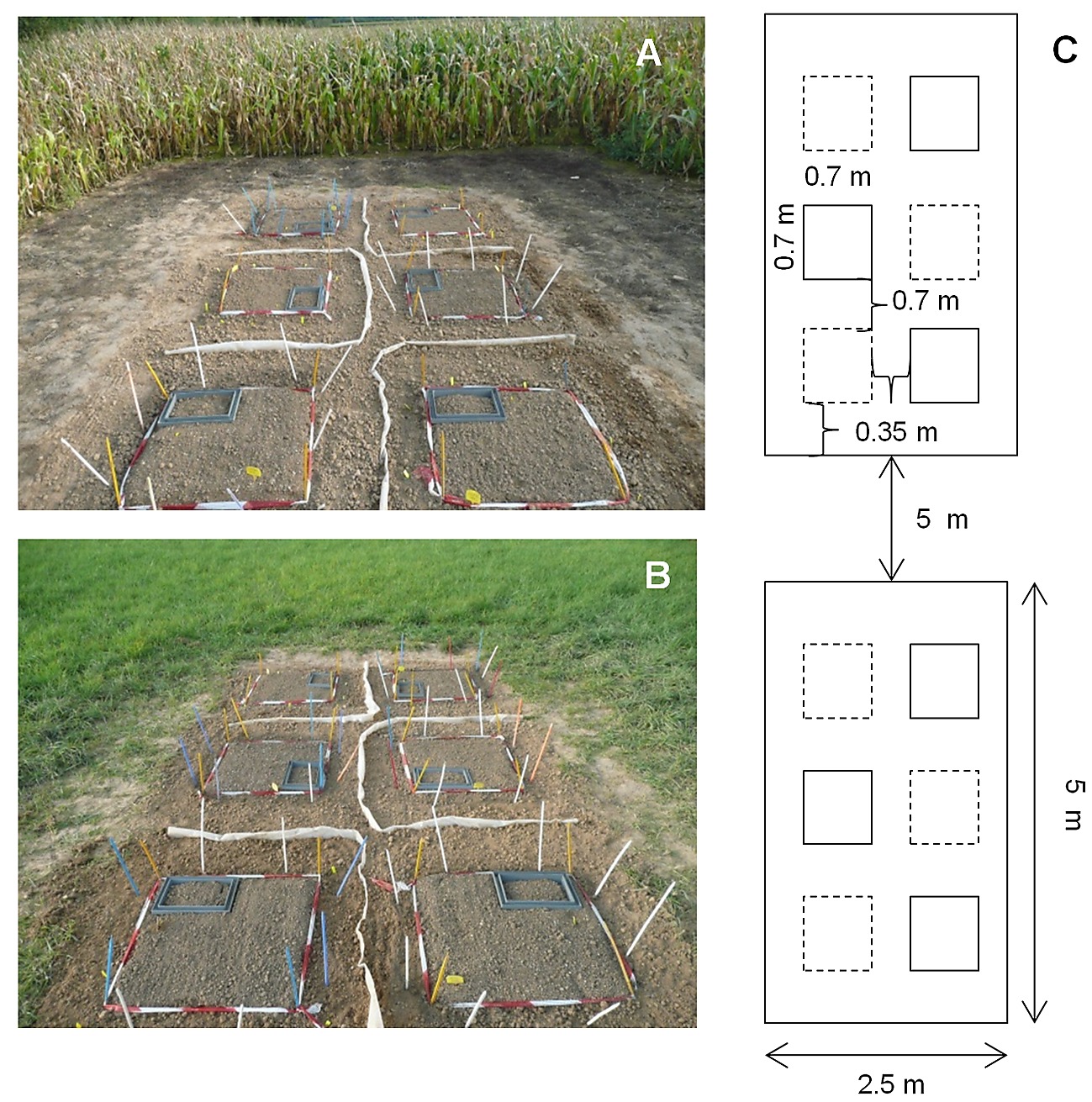
S1 Fig. Organization of the experimental site set up at the SOERE-ACBB (Lusignan, France).
Pictures of the cropland (A), and grassland plot (B) associated with their respective diagrammatic representations (C and D). The two plots (2.5 × 5 m) were separated by a 5 meters pathway. Each plot was divided into 6 subplots of 0.49 m² (0.7 × 0.7), with 3 subplots amended with wheat straw (dotted line), and 3 unamended control subplots (full line). Grey squares in each subplot correspond to the fix part of gaz sampling chambers.
doi:10.1371/journal.pone.0130672.s001
(TIF)
S2 Fig. Heat map comparison of bacterial genera (relative abundance > 1%) detected in soils according to land management history (cropland and grassland) and treatments (control or amended) over time.
In the amended treatment, the white dashed line represents the time of wheat straw input. The legend shows the Z-scores (relative abundances are expressed as median centered Z-scores between all samples, and the colors scaled to standard deviations). For each sampling date, the average of relative abundance was based on the biological replicates (n = 3).
doi:10.1371/journal.pone.0130672.s002
(TIF)
S3 Fig. Heat map comparison of fungal genera (relative abundance > 1%) detected in soils according to land management history (cropland and grassland) and treatments (control or amended) over time.
In the amended treatment, the white dashed line represents the time of wheat straw input. The legend shows the Z-scores (relative abundances are expressed as median centered Z-scores between all samples, and the colors scaled to standard deviations). For each sampling date, the average of relative abundance was based on the biological replicates (n = 3).
doi:10.1371/journal.pone.0130672.s003
(TIF)
S4 Fig. Changes in the climatic parameters at the site during the 1-year period (01/09/2011 to 13/09/2012).
Precipitation (A), soil humidity (black line) and temperature (grey line) (B). All these data were provided by the SOERE-ACBB (http://www.soere-acbb.com/index.php/fr/). Signs (×) indicate time points when soils were sampled for the biological molecular analysis.
doi:10.1371/journal.pone.0130672.s004
(TIF)
Acknowledgments
This work was supported by the Agence Nationale de Recherche (ANR) as part of the ANR Systerra project DIMIMOS (ANR-08-STRA-06) and by a grant from the Regional Council of Burgundy. It benefited from the technical facilities of the GenoSol platform of the infrastructure ANAEE-France [ANAlysis and Experimentations on Ecosystems, ANR project ‘Investments for the Future’ (ANR-11-INBS-0001)], as well as from the ORE-ACBB (http://www.soere-acbb.com/index.php/fr/).
The authors have declared that no competing interests exist.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceived and designed the experiments: PAM LR VT. Performed the experiments: VT PAM CF SD XC CdB. Analyzed the data: VT. Contributed reagents/materials/analysis tools: TR VT ST. Wrote the paper: VT PAM AC TR ST CF SD XC CdB LR.
References
- 1.Swift MJ, Andren O, Brussaard L, Briones M, Couteaux M-M, Ekschmitt K, et al. (1998) Global change, soil biodiversity, and nitrogen cycling in terrestrial ecosystems: three case studies. Glob Change Biol 4: 729–743. doi: 10.1046/j.1365-2486.1998.00207.x
- 2.Mummey D, Holben W, Six J, Stahl P (2006) Spatial stratification of soil bacterial populations in aggregates of diverse soils. Microbial Ecol 51: 404–411. pmid:16598640doi: 10.1007/s00248-006-9020-5
- 3.Torsvik V, Øvreås L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5: 240–245. pmid:12057676 doi: 10.1016/s1369-5274(02)00324-7
- 4.Roesch LFW, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, et al. (2007) Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1: 283–290. pmid:18043639 doi: 10.1038/ismej.2007.53
- 5.Gordon H, Haygarth PM, Bardgett RD (2008) Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol Biochem 40: 302–311. doi: 10.1016/j.soilbio.2007.08.008
- 6.Lauber CL, Ramirez KS, Aanderud Z, Lennon J, Fierer N (2013) Temporal variability in soil microbial communities across land-use types. ISME J 7: 1641–1650. doi: 10.1038/ismej.2013.50. pmid:23552625
- 7.Tardy V, Mathieu O, Lévêque J, Terrat S, Chabbi A, Lemanceau P, et al. (2013) Stability of soil microbial structure and activity depends on microbial diversity. Environ Microbiol Rep 6: 173–183. doi: 10.1111/1758-2229.12126. pmid:24596291
- 8.McMahon SK, Williams MA, Bottomley PJ, Myrold DD (2005) Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. Soil Sci Soc Am J 69: 1238–1247. doi: 10.2136/sssaj2004.0289
- 9.Williams MA, Myrold DD, Bottomley PJ (2007) Carbon flow from 13C-labeled clover and ryegrass residues into a residue-associated microbial community under field conditions. Soil Biol Biochem 39: 819–822. doi: 10.1016/j.soilbio.2006.09.011
- 10.Bastian F, Bouziri L, Nicolardot B, Ranjard L (2009) Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol Biochem 41: 262–275. doi: 10.1016/j.soilbio.2008.10.024
- 11.Baumann K, Marschner P, Smernik RJ, Baldock JA. (2009) Residue chemistry and microbial community structure during decomposition of eucalypt, wheat and vetch residues. Soil Biol Biochem 41: 1966–1975. doi: 10.1016/j.soilbio.2009.06.022
- 12.Marschner P, Umar S, Baumann K (2011) The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol Biochem 43: 445–451. doi: 10.1016/j.soilbio.2010.11.015
- 13.Pascault N, Cécillon L, Mathieu O, Hénault C, Sarr A, Lévêque J, et al. (2010) In situ dynamics of microbial communities during decomposition of wheat, rape, and alfalfa residues. Microbial Ecol 60: 816–828. doi: 10.1007/s00248-010-9705-7. pmid:20593174
- 14.Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S, et al. (2013) Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 16: 810–822. doi: 10.1007/s10021-013-9650-7
- 15.McIntosh RP, Odum EP (1969) Ecological succession. Science 166: 403–404. pmid:17796559 doi: 10.1126/science.166.3903.403-a
- 16.Bernard L, Mougel C, Maron P-A, Nowak V, Lévêque J, Henault C, et al. (2007) Dynamics and identification of soil microbial populations actively assimilating carbon from 13C-labelled wheat residue as estimated by DNA- and RNA-SIP techniques. Environ Microbiol 9: 752–764. pmid:17298374 doi: 10.1111/j.1462-2920.2006.01197.x
- 17.Voříšková J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J 7: 477–486. doi: 10.1038/ismej.2012.116. pmid:23051693
- 18.Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88: 1354–1364. pmid:17601128 doi: 10.1890/05-1839
- 19.De Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29: 795–811. pmid:16102603 doi: 10.1016/j.femsre.2004.11.005
- 20.Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ (2006) Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology 87: 2559–2569. pmid:17089664 doi: 10.1890/0012-9658(2006)87[2559:iobafo]2.0.co;2
- 21.Poll C, Marhan S, Ingwersen J, Kandeler E (2008) Dynamics of litter carbon turnover and microbial abundance in a rye detritusphere. Soil Biol Biochem 40: 1306–1321. doi: 10.1016/j.soilbio.2007.04.002
- 22.Van der Wal A, Geydan TD, Kuyper TW, De Boer W (2013) A thready affair: linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol Rev 37: 477–494. doi: 10.1111/1574-6976.12001. pmid:22978352
- 23.Kim M, Kim W-S, Tripathi BM, Adams J (2014) Distinct bacterial communities dominate tropical and temperate zone leaf litter. Microb Ecol 67: 837–848. doi: 10.1007/s00248-014-0380-y. pmid:24549745
- 24.Acosta-Martínez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40: 2762–2770. doi: 10.1016/j.soilbio.2008.07.022
- 25.Jangid K, Williams MA, Franzluebbers AJ, Sanderlin JS, Reeves JH, Jenkins MB, et al. (2008) Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol Biochem 40: 2843–2853. doi: 10.1016/j.soilbio.2008.07.030
- 26.Jangid K, Williams MA, Franzluebbers AJ, Schmidt TM, Coleman DC, Whitman WB (2011) Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol Biochem 43: 2184–2193. doi: 10.1016/j.soilbio.2011.06.022
- 27.Drenovsky RE, Steenwerth KL, Jackson LE, Scow KM (2010) Land use and climatic factors structure regional patterns in soil microbial communities. Global Ecol Biogeogr 19: 27–39. pmid:24443643 doi: 10.1111/j.1466-8238.2009.00486.x
- 28.Shange RS, Ankumah RO, Ibekwe AM, Zabawa R, Dowd SE (2012) Distinct soil bacterial communities revealed under a diversely managed agroecosystem. PloS ONE 7: e40338. doi: 10.1371/journal.pone.0040338. pmid:22844402
- 29.Pascault N, Nicolardot B, Bastian F, Thiébeau P, Ranjard L, Maron PA (2010) In situ dynamics and spatial heterogeneity of soil bacterial communities under different crop residue management. Microbial Ecol 60: 291–303. doi: 10.1007/s00248-010-9648-z. pmid:20352206
- 30.Baumann K, Dignac M-F, Rumpel C, Bardoux G, Sarr A, Steffens M (2012) Soil microbial diversity affects soil organic matter decomposition in a silty grassland soil. Biogeochemistry 114: 201–212. doi: 10.1007/s10533-012-9800-6
- 31.Wagg C, Bender SF, Widmer F, Van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. P Natl Acad Sci USA 111: 5266–5270. doi: 10.1073/pnas.1320054111. pmid:24639507
- 32.Chabbi A, Kögel-Knabner I, Rumpel C (2009) Stabilised carbon in subsoil horizons is located in spatially distinct parts of the soil profile. Soil Biol Biochem 41: 256–261. doi: 10.1016/j.soilbio.2008.10.033
- 33.Terrat S, Christen R, Dequiedt S, Lelièvre M, Nowak V, Regnier T, et al. (2012) Molecular biomass and MetaTaxogenomic assessment of soil microbial communities as influenced by soil DNA extraction procedure. Microbial biotechnology 5: 135–141. doi: 10.1111/j.1751-7915.2011.00307.x. pmid:21989224
- 34.Dequiedt S, Saby NPA, Lelievre M, Jolivet C, Thioulouse J, Toutain B, et al. (2011) Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Global Ecol Biogeogr 20: 641–652. doi: 10.1111/j.1466-8238.2010.00628.x
- 35.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic acids research 37: D141–145. doi: 10.1093/nar/gkn879. pmid:19004872
- 36.Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microb 71: 8228–8235. pmid:16332807 doi: 10.1128/aem.71.12.8228-8235.2005
- 37.Orgiazzi A, Lumini E, Nilsson RH, Girlanda M, Vizzini A, Bonfante P, et al. (2012) Unravelling soil fungal communities from different Mediterranean land-use backgrounds. PloS ONE 7: e34847. doi: 10.1371/journal.pone.0034847. pmid:22536336
- 38.Bucldey DH, Schmidt TM (2001) The structure of microbial communities in soil and the lasting impact of cultivation. Microb Ecol 42: 11–21. pmid:12035077
- 39.McLauchlan K (2006) The nature and longevity of agricultural impacts on soil carbon and nutrients: a review. Ecosystems 9: 1364–1382. doi: 10.1007/s10021-005-0135-1
- 40.Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Natl Acad Sci USA 103: 626–631. pmid:16407148 doi: 10.1073/pnas.0507535103
- 41.Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40: 2407–2415. doi: 10.1016/j.soilbio.2008.05.021
- 42.Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12: 1238–1249. doi: 10.1111/j.1461-0248.2009.01360.x. pmid:19674041
- 43.Ranjard L, Dequiedt S, Chemidlin Prévost-Bouré N, Thioulouse J, Saby NPA, Lelièvre M, et al. (2013) Turnover of soil bacterial diversity driven by wide-scale environmental heterogeneity. Nat Commun 4: 1434. doi: 10.1038/ncomms2431. pmid:23385579
- 44.Smith P (2008) Land use change and soil organic carbon dynamics. Nutr Cycl Agroecosyst 81: 169–178. doi: 10.1007/s10705-007-9138-y
- 45.Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N (2009) A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3: 442–453. doi: 10.1038/ismej.2008.127. pmid:19129864
- 46.Naether A, Foesel BU, Naegele V, Wüst PK, Weinert J, Bonkowski M, et al. (2012) Environmental factors affect Acidobacterial communities below the subgroup level in grassland and forest soils. Appl Environ Microb 78: 7398–7406. pmid:22885760 doi: 10.1128/aem.01325-12
- 47.DeBruyn JM, Nixon LT, Fawaz MN, Johnson AM, Radosevich M (2011) Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl Environ Microb 77: 6295–6300. doi: 10.1128/AEM.05005-11. pmid:21764958
- 48.De Castro AP, Quirino BF, Pappas G, Kurokawa AS, Neto EL, Krüger RH (2008) Diversity of soil fungal communities of Cerrado and its closely surrounding agriculture fields. Arch Microbiol 190: 129–139. doi: 10.1007/s00203-008-0374-6. pmid:18458875
- 49.Klaubauf S, Inselsbacher E, Zechmeister-Boltenstern S, Wanek W, Gottsberger R, et al. (2010) Molecular diversity of fungal communities in agricultural soils from Lower Austria. Fungal Diversity 44: 65–75. pmid:23794962 doi: 10.1007/s13225-010-0053-1
- 50.Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, et al. (2009) 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol 184: 449–456. doi: 10.1111/j.1469-8137.2009.03003.x. pmid:19703112
- 51.Anderson IC, Campbell CD, Prosser JI (2003) Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ Microbiol 5: 36–47. pmid:12542711 doi: 10.1046/j.1462-2920.2003.00383.x
- 52.Lienhard P, Terrat S, Prévost-Bouré NC, Nowak V, Régnier T, Sayphoummie S, et al. (2013) Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron Sustain Dev doi: 10.1007/s13593-013-0162-9.
- 53.Paterson E, Osler G, Dawson LA, Gebbing T, Sim A, Ord B (2008) Labile and recalcitrant plant fractions are utilised by distinct microbial communities in soil: Independent of the presence of roots and mycorrhizal fungi. Soil Biol Biochem 40: 1103–1113. doi: 10.1016/j.soilbio.2007.12.003
- 54.De Deyn GB, Quirk H, Oakley S, Ostle N, Bardgett RD (2011) Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed species-rich grasslands. Biogeosciences 8: 1131–1139. doi: 10.5194/bg-8-1131-2011
- 55.Hannula SE, Boschker HTS, De Boer W, Van Veen JA (2012) 13C pulse-labeling assessment of the community structure of active fungi in the rhizosphere of a genetically starch-modified potato (Solanum tuberosum) cultivar and its parental isoline. New Phytol 194: 784–799. doi: 10.1111/j.1469-8137.2012.04089.x. pmid:22413848
- 56.Aneja MK, Sharma S, Fleischmann F, Stich S, Heller W, Bahnweg G, et al. (2006) Microbial colonization of beech and spruce litter--influence of decomposition site and plant litter species on the diversity of microbial community. Microbial Ecol 52: 127–135. pmid:16691328 doi: 10.1007/s00248-006-9006-3
- 57.Jenkins SN, Rushton SP, Lanyon CV, Whiteley AS, Waite IS, et al. (2010) Taxon-specific responses of soil bacteria to the addition of low level C inputs. Soil Biol Biochem 42: 1624–1631. doi: 10.1016/j.soilbio.2010.06.002
- 58.Ofek M, Hadar Y, Minz D (2012) Ecology of root colonizing Massilia(Oxalobacteraceae). PloS ONE 7: e40117. doi: 10.1371/journal.pone.0040117. pmid:22808103
- 59.Suárez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microbial Ecol 63: 249–266. doi: 10.1007/s00248-011-9929-1. pmid:21850446
- 60.Postma J, Schilder MT, Van Hoof RA (2011) Indigenous populations of three closely related Lysobacter spp. in agricultural soils using real-time PCR. Microbial Ecol 62: 948–958. doi: 10.1007/s00248-011-9847-2. pmid:21448673
- 61.Deacon JW (2006) Fungal Biology 4th edition. Blackwell Publishing Ltd. 380 p.
- 62.Richardson M (2009) The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infec 15: 2–9. doi: 10.1111/j.1469-0691.2009.02972.x
- 63.Eida MF, Nagaoka T, Wasaki J, Kouno K (2011) Evaluation of cellulolytic and hemicellulolytic abilities of fungi isolated from coffee residue and sawdust composts. Microbes and Environ 26: 220–227. pmid:21558674 doi: 10.1264/jsme2.me10210
- 64.Kumar R, Sinha A, Srivastava S, Srivastava M (2011) Variation in soil mycobiota associated with decomposition of sesbania aculeta L. Asian J Plant Pathology 5: 37–45. doi: 10.3923/ajppaj.2011.37.45
- 65.Silvestro LB, Stenglein SA, Forjan H, Dinolfo MI, Arambarri AM (2013) Occurrence and distribution of soil Fusarium species under wheat crop in zero tillage. Span J Agric Res 11: 72–79. doi: 10.5424/sjar/2013111-3081
- 66.Wakelin SA, Warren RA, Kong L, Harvey PR (2008) Management factors affecting size and structure of soil Fusarium communities under irrigated maize in Australia. Appl Soil Ecol 39: 201–209. doi: 10.1016/j.apsoil.2007.12.009
For further details log on website :
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0130672





No comments:
Post a Comment