At the heart of modern chemistry lies an assumption, a model really, that all chemists take for granted. The assumption had its origin with the ancient Greeks, such as Democritus, who enjoyed speculating about the nature of the universe while sipping their wine at the dinner table. The word atoms comes from the Greek, atomos, meaning uncuttable. According to some Greek philosophers, the universe consists of tiny pieces of matter that themselves cannot be further subdivided. Thus, a piece of paper can be torn into many small pieces and with a sharp knife can be further divided into even smaller pieces, but at some point the cutting must stop. At this point, the pieces would be extremely small, and these were thought to be the building blocks from which all matter was built. Another school of thought, the Aristotelian group, believed that matter was infinitely divisible, that one could take the smallest piece of matter imaginable and then divide it into smaller pieces, and in turn divide those pieces, and on and on. Although the atomic school of thought is the one that dominates all of science, it may come as a surprise to you that this matter is probably by no means settled. The theoretical physicists have found numerous particles that are pieces of the atom, and seem to find more every year. [At the moment many physicists believe that one of these particles, the quark, is the fundamental particle from which all other matter is constructed.


http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/matter-at-the-atomic-level/atoms
The Greeks were not experimentalists; it was not until about the tenth century and the beginning of Alchemy that chemistry progressed as we think of it today; that is, as an experimental science. By the 18th century scientists had done thousands of experiments on the properties of matter and had formulated laws such as the Law of Conservation of Matter, and the Law of Multiple Proportions. Moreover, it was known that matter can be divided into mixtures and substances, with substances being either compounds or elements (see Figure 6). Compounds can be separated into their constituent elements, but elements cannot be further subdivided. Thus, atoms are the building blocks of matter on the atomic scale, elements are the building blocks of matter on the macroscopic level.

Figure 6. The classification of matter.
These laws allowed John Dalton, an English school teacher, to formulate a more modern version of the atomic model. Dalton was able to explain these and other Laws by making the following assumptions:
- All matter consists of small, indivisible particles (atoms).
- All of the atoms of the same element are the same; that is, have the same size, weight, color, etc. Atoms of different elements are different.
- Compounds consist of combinations of atoms of different elements in whole number ratios.
For example, according to Dalton, common table salt (sodium chloride) consists of a one to one ratio of sodium atoms to chlorine atoms. In fact, Dalton probably visualized sodium chloride pretty much as shown in Figure 7.

Figure 7. Dalton's view of sodium chloride.
Dalton's atomic theory helped chemists to understand the laws that had been formulated and many of the observations that had been made. Like most theories, however, within 50 years it was shown to be wrong in several respects.
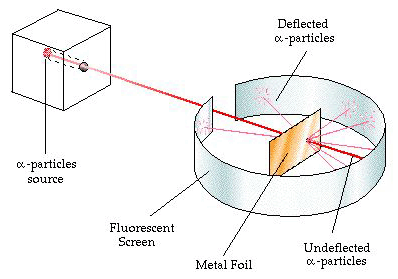
Around the turn of the century, chemists found evidence that atoms are not the solid, indivisible entities that Dalton imagined. The three particles that "live" inside the atom are: a) the electron, a tiny, negatively charged particle that controls how atoms bond to one another, b) the proton, a much heavier, positively charged particle, and c) the neutron, a particle of essentially the same mass as the proton, but without a charge. After these subatomic particles were discovered, chemists and physicists naturally speculated about the structure of the atom. How were the electrons and protons arranged? After all, electrons and protons are oppositely charged and should be strongly attracted to one another. One hypothesis was that the electrons and protons were evenly distributed throughout the atom. This was disproved by experiments by Rutherford in the early part of this century, in which he directed alpha particles (positively charged particles that had been discovered in naturally-occuring radioactivity) toward a thin metal sheet (see Figure 8). Some of the alpha particles were deflected back to the source in a way that could only be explained by a high concentration of positive charge in the center of the atom.

Figure 8. The Rutherford alpha particle scattering experiment.
Rutherford's hypothesis, then, was that all of the protons and neutrons reside in a very small center or nucleus of the atom and that the electrons are outside of the nucleus. If an atom were the size of a football field, the nucleus would be about the size of a baseball at the center of the field, and the electrons would be the size of grains of sand around the outside of the field. Clearly, according to this, the currently accepted model, most of the atom is empty space.
One of the most important consequences of the discovery of the subatomic particles was the realization that the atoms of different elements contain different numbers of protons. For example, all atoms of carbon contain six protons, all atoms of oxygen contain eight protons. The number of protons in the nucleus of an atom is called the atomic number. A portion of the periodic table of the elements is shown in Figure 9. Notice that in this table the atomic number is given above the symbol for each element; the elements are arranged in order of increasing atomic number from left to right and down the table.
| 1 | 18 | ||||||||||||||||
| 1 H 1.0079 | 2 | 13 | 14 | 15 | 16 | 17 | 2 He 4.0026 | ||||||||||
| 3 Li 6.941 | 4 Be 9.0122 | 5 B 10.811 | 6 C 12.011 | 7 N 14.007 | 8 O 15.999 | 9 F 18.998 | 10 Ne 20.180 | ||||||||||
| 11 Na 22.990 | 12 Mg 24.305 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 Al 26.982 | 14 Si 28.086 | 15 P 30.974 | 16 S 32.065 | 17 Cl 35.453 | 18 Ar 39.948 |
| 19 K 39.098 | 20 Ca 40.078 | 21 Sc 44.956 | 22 Ti 47.867 | 23 V 50.942 | 24 Cr 51.996 | 25 Mn 54.938 | 26 Fe 55.845 | 27 Co 58.933 | 28 Ni 58.693 | 29 Cu 63.546 | 30 Zn 65.409 | 31 Ga 69.723 | 32 Ge 72.64 | 33 As 74.922 | 34 Se 78.96 | 35 Br 79.904 | 36 Kr 83.798 |
| 37 Rb 85.468 | 38 Sr 87.62 | 39 Y 88.906 | 40 Zr 91.224 | 41 Nb 92.906 | 42 Mo 95.94 | 43 Tc (98) | 44 Ru 101.07 | 45 Rh 102.91 | 46 Pd 106.42 | 47 Ag 107.87 | 48 Cd 112.41 | 49 In 114.82 | 50 Sn 118.71 | 51 Sb 121.76 | 52 Te 127.60 | 53 I 126.90 | 54 Xe 131.29 |
| 55 Cs 132.91 | 56 Ba 137.33 | 57 - 71 | 72 Hf 178.49 | 73 Ta 180.95 | 74 W 183.84 | 75 Re 186.21 | 76 Os 190.23 | 77 Ir 192.22 | 78 Pt 195.08 | 79 Au 196.97 | 80 Hg 200.59 | 81 Tl 204.38 | 82 Pb 207.2 | 83 Bi 208.98 | 84 Po (209) | 85 At (210) | 86 Rn (222) |
| 87 Fr (223) | 88 Ra (226) | 89 - 103 | 104 Rf (261) | 105 Db (262) | 106 Sg (266) | 107 Bh (264) | 108 Hs (277) | 109 Mt (268) | 110 Ds (271) | 111 Rg (272) | |||||||
| 57 La 138.91 | 58 Ce 140.12 | 59 Pr 140.91 | 60 Nd 144.24 | 61 Pm (145) | 62 Sm 150.36 | 63 Eu 151.96 | 64 Gd 157.25 | 65 Tb 158.93 | 66 Dy 162.50 | 67 Ho 164.93 | 68 Er 167.26 | 69 Tm 168.93 | 70 Yb 173.04 | 71 Lu 174.97 | |||
| 89 Ac (227) | 90 Th 232.04 | 91 Pa 231.04 | 92 U 238.03 | 93 Np (237) | 94 Pu (244) | 95 Am (243) | 96 Cm (247) | 97 Bk (247) | 98 Cf (251) | 99 Es (252) | 100 Fm (257) | 101 Md (258) | 102 No (259) | 103 Lr (262) | |||
Figure 9. The periodic table of the elements.
Problem One
How many protons are there in the nucleus of an atom of the element calcium (Ca)?
With the invention of instrumentation such as the mass spectrometer, which allows atoms of different mass to be separated and identified, another discovery was made: atoms of the same element can have different numbers of neutrons. For example, there are two naturally-occurring carbon atoms: one has 6 neutrons, the other has 7 neutrons. The total number of protons and neutrons is called the mass number. Two atoms of the same element that contain different numbers of neutrons are called isotopes. The mass number of an element is frequently designated as a superscript on the left side of the symbol for the element. Thus, 13C is the isotope of carbon that contains 7 neutrons. Sometimes, the atomic number and the mass number are designated at the same time, the atomic number as a subscript and the mass number as a superscript.
The number of electrons in an atom is determined by the charge on the atom. If the atom is neutral, that is, has no charge, then the positive charge produced by the protons must be balanced by the negative charge of the electrons. Thus, a neutral carbon atom must contain six electrons. However, if the carbon atom has a positive charge of one it will contain one less electron. Positively charged atoms have more protons than electrons and are called cations. Negatively charged atoms have more electrons than protons and are called anions. The charge of an atom is designated by a superscript on the right hand side of the symbol of the element. A carbon atom that has eight electrons would be designated as C2-.
Problem Two
What is the symbol for the ion with an atomic number of 12 that has 10 electrons?
In addition to the atomic number, the atomic weight of each element is also given, located below the symbol for the element on the periodic chart. Generally, chemists measure the mass of things in grams or kilograms (a kilogram is a thousand grams, a milligram is a thousandth of a gram). In fact, almost everything that a scientist measures has units; if speed is being measured, the units may be meters per second (m/s); if volume is measured it is expressed as cubic meters, or cubic centimeters (a cubic centimeter is a milliliter). Without these units the scientific community would have no idea of what the number designated. Suppose for example, that a chemist reports that 32 of sulfur is mixed with 23 of magnesium. What is the chemist referring to? Volume--if so does she mean 32 milliliters (mL) or 32 liters? Or is it mass--if so, is it 32 grams (g) of sulfur or 32 milligrams? It could even be 32 pounds of sulfur if the chemist does not use the metric system. Because these units are so important, scientists have agreed to use the metric system of measurement and prefixes for the metric units that are shown in Table 1. In certain applications, non metric units may still be used, however. In 1960, the General Conference of Weights and Measurements recommended that a single unit be used for each measured quantity. This Systeme International d' Unites (International System of Units, SI) is based on the metric system and consists of the seven fundamental base units shown in Table 2.
| Factor | Prefix | Symbol |
|---|---|---|
| 109 | giga | G |
| 106 | mega | M |
| 103 | kilo | k |
| 10-1 | deci | d |
| 10-2 | centi | c |
| 10-3 | milli | m |
| 10-6 | micro | μ |
| 10-9 | nano | n |
| 10-12 | pico | p |
| 10-15 | femto | f |
| Quantity | Name of Unit | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Electric current | ampere | A |
| Thermodynamic temperature | kelvin | K |
| Luminous intensity | candela | cd |
| Amount of substance | mole | mol |
For example, 1 MeV = 106 electron volts; 1 pm = 10-12 meters; 1 mg = 10-3 grams.
Other units can be derived from the SI base units. For example, the SI unit for volume is cubic meters (m3); the SI unit for force is the newton, N, which is obtained when mass is expressed in kilograms, and acceleration is given in meters per second; pressure is given in pascals (Pa), which is a newton per square meter. The complete adoption of SI units has not yet occurred. Pressure, for example, is usually expressed as torr, millimeters of mercury, or atmospheres (in some cases even in the English units of pounds per square inch, psi). Likewise, volume is usually expressed in liters (L) which is 1000 cm3; temperature is often given in degrees Celsius ( or centigrade, °C) or even in the English units of Fahrenheit (°F).
At any rate, the atomic weight given below the element in the periodic chart does not contain a unit. Of course, this could simply be a result of a decision on the part of the illustrator, an assumption, perhaps, that everyone would know that the weight was given in units of, say, tons. In fact, these weights really do not have units, they are simply relative. Hundreds of chemists worked for many years to establish these relative atomic weights. Originally, the weights were adjusted so that hydrogen, the lightest element, would have a weight of 1. If you compare hydrogen and oxygen, you will notice that an atom of oxygen is 16 times heavier than an atom of hydrogen. This arbitrary scale was adjusted later on, after the invention of the mass spectrometer and the discovery of isotopes. The weight of the 12C nucleus was then assigned a value of exactly 12 (12.00000). Notice that because there is a little 13C present in carbon, the atomic weight of carbon is not 12.000000, but 12.01.
As you might imagine someone started to wonder about the absolute mass of atoms. How much does one 12C atom weigh? This weight can be expressed as 12.0000 atomic mass units (amu), but unless we know how much an atomic mass unit is, this is not particularly helpful. This problem was solved by determining the number of atoms of an element in an amount of the element equal to its atomic weight in grams. This number, called Avogadro's number in honor of the famous Italian scientist, Amadeo Avogadro, is huge--602200000000000000000000. Because numbers such as this are very cumbersome to read they are usually expressed in scientific notation. This number is given as 6.022 x 1023, which means that 6.022 is multiplied by 10 raised to the 23rd power; in other words, that 6.022 is multiplied by a 1 followed by 23 zeros. The magnitude of this number can be appreciated by imagining a gigantic wall constructed of Avogadro's number of grains of sand (each 0.05 cm in diameter). If this wall were one mile wide and one mile high, it would extend all the way around the earth (about 20,000 miles).
Problem Three
If you are not familiar with scientific notations, sometimes also called exponential notation, take another look at 6 x 1023. It literally means that you multiply 6 by a 1 with 23 zeros to the right of it. If a number is expressed with a negative exponent; for example, 3.1 x 10-3, means that 3.1 is multiplied by 1 with 3 places between it and the decimal place -- 0.001. When you multiply 3.1 by 0.001 you get 0.0031. Thus, 3.1 x 10-3 is the same as 0.0031. Can you express 2.23 x 102 and 6.02 x 10-5 in their decimal equivalents?
Phosphorus has an atomic weight of 31.0. In this 31.0 grams there are 6 x 1023atoms. Clearly, one average atom of phoshorus must have a very tiny mass. Indeed, we can calculate this mass by dividing the mass of 6.02 x 1023 atoms (31.0 g) by the number of atoms:
(31.0 g)/(6 x 1023) = 5 x 10-23 g
Thus, one atom of phosphorus weighs 5 x 10-23 g, a very tiny mass indeed.
Avogadro's number of atoms is so frequently used that it has been given a special name-- a mole. This term is similar to the use of dozen for 12 items or ream for 500 sheets of paper. Thus, in 31 grams of phosphorus there is a mole of phosphorus atoms (6 x 1023 atoms). Five moles of carbon is 5 x 12 g = 60 grams of carbon. This 60 grams of carbon contains 5 x (6 x 1023) = 30 x 1023 atoms. Chemists are so accustomed to working with moles that you will hear them say things like: "The reaction was run on a 0.1 mole scale", or "We'll need at least a millimole in order to obtain a good spectrum."
For further information log on website :http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/matter-at-the-atomic-level/atoms





No comments:
Post a Comment