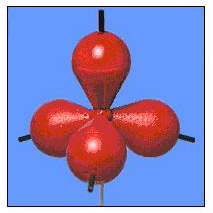
Not long after G.N. Lewis popularized the electron-dot model, quantum mechanics became a very powerful model, including its use to describe the chemical bond. Two models--the valence bond model and the molecular orbital model--were developed almost simultaneously. Linus Pauling became the champion of the valence bond model, which is easier to visualize and use. This model is essentially a quantum mechanical version of the electron-dot model: it attempts to describe what orbitals are used by each atom when electrons are shared. For example, when the simple molecule H2 is formed from hydrogen atoms, the valence bond model says that an s-orbital on one atom overlaps with an s-orbital on the other to form a bond. This is sometimes depicted by an overlap diagram and a picture as shown in Figure 30.




http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/compounds/valence-bond-and-molecular-orbital-models

Figure 30. Overlap diagram and picture for the valence bond description of diatomic hydrogen.
One of the problems that Pauling encountered early in his pioneering efforts with valence bond theory is clearly exemplified by a simple valence bond picture of the bonding in water. Figure 31 shows two p-orbitals on oxygen overlapping with s-orbitals on hydrogens. This picture shows clearly that the bond angle in water should be 90°, because the two p-orbitals are aligned along the x and y axes and are therefore perpendicular.

Figure 31. Orbital overlap in water.
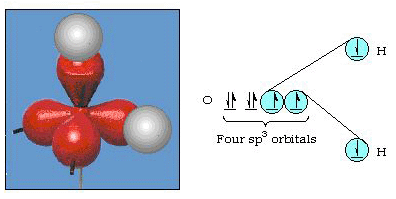
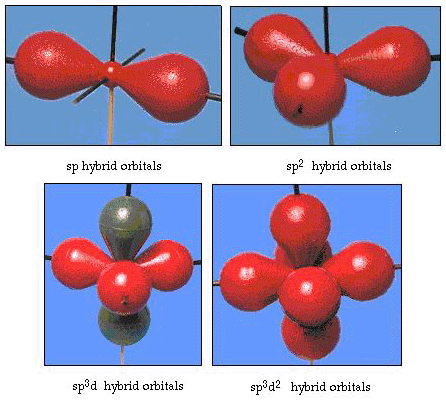
In fact, the bond angle in water is 105°, a significant departure from 90°. This kind of error might not seem significant to you, but to someone trying to formulate a model that will explain as much of a certain part of nature as possible, this difference between theory and experimental fact is very bothersome. Pauling then added another modification to the theory--hybridization. Pauling said that atomic orbitals on an atom are not sacrosanct, they are just one possible set of mathematical equations (remember our discussion of the wave model) that can be used to describe the electrons in that atom. Other sets can be generated by adding and subtracting these atomic orbitals according to certain rules. Thus, if an s orbital and the three p-orbitals are combined mathematically, a new set of orbitals, sp3orbitals, are generated. The new set of orbitals has different orientational characteristics than the set of atomic orbitals. Figure 32 shows the s and three p-orbitals in part (a) and then in part (b) the four new sp3 hybrids. As you can see, these sp3 hybrids are oriented toward the corners of a tetrahedron. Figure 33 shows these hybrids being used to describe the bonding in water. Because the angle between the hybrids is 109°, we now expect the bond angle in water to be 109°. Clearly, this gives us better agreement with the experimental value of 105°. (Notice that this is not in perfect agreement, but it is the best we can do.) Other hybridization combinations are also possible, and these are summarized in Figure 34.

Figure 32. sp3 hybridization.

Figure 33. Use of sp3 hybrids to explain the bond angle in water.

Figure 34. Other hybridization schemes.
press for video
press for video
There is one other feature of the valence bond model that deserves discussion. It is nicely illustrated by our familiar oxygen molecule, O2. Figure 35 shows the overlap of two px-orbitals to give us a single bond. If we are to obey the octet rule, we must also form another bond. This must be by overlap of two py or two pz orbitals (it doesn't matter which). This bond has its electron density above and below the internuclear line, rather than directly between the two oxygen nuclei. This bond is referred to as a 1-bond, whereas the one that puts electron density between the atoms is called a sigma (σ) bond. One of the interesting consequences of this picture is that the 1-bond is anticipated to be weaker than the sigma-bond (the overlap of the orbitals is weaker). This means that a double bond does not have the strength of two single bonds and is also more susceptible to attack by external atoms that are searching for electron density.

Figure 35. Formation of sigma and pi bonds in diatomic oxygen.
As a final example of the valence bond treatment of bonding, Figure 36 shows a picture of the orbitals involved in the bonding in ethene, the simplest unsaturated hydrocarbon (H2C=CH2). Experimentally, it has been shown that the bond angles around each carbon are nearly 120°. Thus, our picture shows the use of sp2 hybrids (because they have 120° angles between each other) for the construction of the sigma bonds. The 1-bond is formed by overlap of two pz orbitals. It is important to notice that the 1-bond introduces a barrier to rotation about the carbon-carbon linkage. That is, if one CH2 group were to rotate, the 1-bond would have to be broken, which would require considerable energy. The consequence of this is that the 1-bond fixes the two CH2 groups in one plane, causing the overall molecule to be planar.

Figure 36. The bonding in ethene.
The molecular orbital model takes a different approach. It utilizes all of the orbitals on all of the atoms to generate a set of orbitals that extend over all of the atoms on the whole molecule. The molecular orbital model is more difficult to visualize, but it is also more powerful. The difficult process of determining how to combine atomic obitals to generate molecular orbitals is now done by computer for even large molecules. The practicing chemist uses the molecular orbital model to predict spectra, transition states, etc., but for everyday chemical discussions, the electron-dot and valence bond models still rule the day.
For further information log on website :http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/compounds/valence-bond-and-molecular-orbital-models





No comments:
Post a Comment