We can now return to compounds that differ only in their 3-dimensional structures. Geometric isomers have the same structural formulas but differ in the arrangement of groups at a single atom, at double bonds, or in rings. Cis- and trans-platin (see Figure 37) are examples of geometric isomers based on the different arrangement of groups at a single atom. Cis- and trans-2-butene differ in the arrangement of the methyl groups about the double bonds.



 For further information log on website :
For further information log on website :
http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/structure/geometric-and-optical-isomers

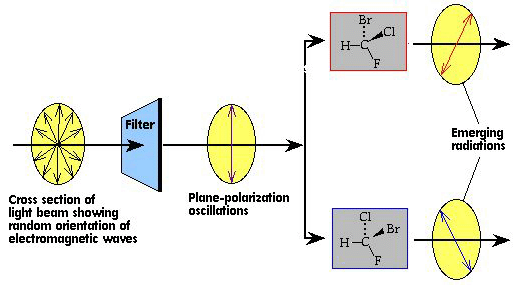
Although geometric isomers have completely different physical and chemical properties (for example, cis- and trans-2-butene have different boiling points and densities), optical isomers (also called enantiomers) differ in only one characteristic--their interaction with plane polarized light. When a beam of light is passed through a certain type of filter, all of the waves except those in one plane are removed. Figure 39 shows this plane-polarized light impinging upon and being rotated by two optical isomers. One of the optical isomers rotates the light in one direction, the other rotates the light in the opposite direction but by the same amount. In every other way, such as boiling point, density, refractive index, viscosity, etc., the two optical isomers are identical.

Figure 39. The interaction of optical isomers with plane-polarized light.
What are these optical isomers? Optical isomers are mirror images that are not superimposable. Your hands, assuming that we do not examine them too closely for scratches and other imperfections, are nonsuperimposable mirror images. Imagine that you approach a mirror and raise your right hand in greeting so that you see the palm of your right hand in the mirror. What you see in the mirror is the mirror image of your right hand and it looks exactly like the palm of your left hand. So your left hand is a mirror image of your right hand. Now, try to superimpose your two hands by laying one atop the other with the palms facing in the same direction. Notice that no matter how you twist them, they cannot be oriented so that the right thumb is on top of the left thumb, the right little finger on top of the left little finger, etc.
If you now perform the same exercise with a pencil, you will notice that the mirror image of a pencil is superimposable on the pencil. Clearly, there must be something about the symmetry of your hands that cause them to be nonsuperimposable.
On the molecular scale, most optical isomers are organic compounds, and they have non superimposable mirror images when there are four different groups on a carbon (the carbon is said to be chiral, from the Greek meaning "handed"). Amino acids are good examples of molecules that have nonsuperimposable mirror images. Alanine has the structural formula

and the two mirror images shown in Figure 40. The peptides and proteins that make up much of our bodies are constructed from combinations of amino acids like alanine. One of the fascinating aspects of chemical evolution is the fact that only one optical isomer is used by natural organisms in producing biological materials. It is also frequently the case that optical isomers differ in their biological activity. We have already seen this in part I during our discussion of levorphane and dextrophane.

Figure 40. Mirror images of the amino acid alanine.
As one further example of optical activity, consider the compound carvone. The optical isomers are shown in Figure 41. The one on the left is responsible for the odor and taste of caraway and dill, the one on the right is isolated from spearmint.

Figure 41. The optical isomers of carvone.
http://www.wiredchemist.com/chemistry/instructional/an-introduction-to-chemistry/structure/geometric-and-optical-isomers






No comments:
Post a Comment