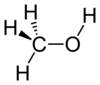
Methanol, also known as methyl alcohol,wood alcohol, wood naphtha, methyl hydrate, or wood spirits, is a chemicalwith the formula CH3OH (often abbreviated MeOH). Methanol acquired the name "wood alcohol" because it was once produced chiefly as a byproduct of the destructive distillation of wood. Today, industrial methanol is produced in a catalytic process directly from carbon monoxide, carbon dioxide, and hydrogen.
Methanol is the simplest alcohol, being only a methyl group linked to a hydroxylgroup. It is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to that of ethanol (drinking alcohol).[11] However, unlike ethanol, methanol is highly toxic and unfit for consumption. At room temperature, it is a polar liquid, and is used as an antifreeze, solvent, fuel, and as a denaturant for ethanol. It is also used for producing biodiesel via transesterification reaction.
Methanol is produced naturally in the anaerobic metabolism of many varieties of bacteria, and is commonly present in small amounts in the environment. As a result, the atmosphere contains a small amount of methanol vapor. But in only a few days, atmospheric methanol is oxidized by sunlight to produce carbon dioxide and water.
Methanol is also found in abundant quantities in star forming regions of space, and is used in astronomy as a marker for such regions. It is detected through its spectral emission lines.[12]
Methanol burns in oxygen, including open air, forming carbon dioxide and water:
- 2 CH3OH + 3 O2 → 2 CO2 + 4 H2O
Methanol ingested in large quantities is metabolized first to formaldehyde and then to formic acid[13] or formate salts, which are poisonous to the central nervous system and may cause blindness, coma, and death. Because of these toxic properties, methanol is frequently used as a denaturant additive for ethanol manufactured for industrial uses. This addition of methanol exempts industrial ethanol (commonly known as "denatured alcohol" or "methylated spirit") from liquor excise taxation in the US and some other countries.
Occurrence
Human Metabolite
Methanol is poisonous to the central nervous system and may cause blindness, coma, and death. However, in small amounts, methanol is a natural endogenous compound found in normal, healthy human individuals. A study found a mean of 4.5 ppm in the exhaled breath of subjects.[14] The mean endogenous methanol production in humans of 0.45 g/d may be metabolized from pectin found in fruit; one kilogram of apple produces up to 1.4 g methanol.[15]
Toxicity
Methanol has a high toxicity in humans. As little as 10 mL of pure methanol, ingested, is metabolized into formic acid, which can cause permanent blindness by destruction of the optic nerve. 30 mL is potentially fatal,[16] although the median lethal dose is typically 100 mL (3.4 fl oz) (i.e. 1–2 mL/kg body weight of pure methanol[17]). Reference dose for methanol is 2 mg/kg/day.[18] Toxic effects begin hours after ingestion, and antidotes can often prevent permanent damage.[16] Because of its similarities in both appearance and odor to ethanol(the alcohol in beverages), it is difficult to differentiate between the two (such is also the case with denatured alcohol). However, there are cases of methanol resistance, such as that of Mike Malloy who was the victim of a failed murder attempt by methanol in the early 1930s.[19]
Methanol is toxic by two mechanisms. First, methanol (whether it enters the body by ingestion, inhalation, or absorption through the skin) can be fatal due to its CNS depressantproperties in the same manner as ethanol poisoning. Second, in a process of toxication, it is metabolized to formic acid (which is present as the formate ion) via formaldehyde in a process initiated by the enzyme alcohol dehydrogenase in the liver.[20] Methanol is converted to formaldehyde via alcohol dehydrogenase (ADH) and formaldehyde is converted to formic acid (formate) via aldehyde dehydrogenase (ALDH). The conversion to formate via ALDH proceeds completely, with no detectable formaldehyde remaining.[21] Formate is toxic because it inhibits mitochondrial cytochrome c oxidase, causing hypoxia at the cellular level, and metabolic acidosis, among a variety of other metabolic disturbances.[22]
Methanol poisoning can be treated with fomepizole, or if unavailable, ethanol.[20][23][24] Both drugs act to reduce the action of alcohol dehydrogenase on methanol by means of competitive inhibition, and the methanol is excreted by the kidneys rather than transformed into toxic metabolites.[20] Additional treatment may include sodium bicarbonate for metabolic acidosis, and hemodialysis or hemodiafiltration to remove methanol and formate from the blood.[20] Folinic acid or folic acid is also administered to enhance the metabolism of formate.[20]
The initial symptoms of methanol intoxication include central nervous system depression, headache, dizziness, nausea, lack of coordination, and confusion. Sufficiently large doses cause unconsciousness and death. The initial symptoms of methanol exposure are usually less severe than the symptoms from the ingestion of a similar quantity of ethanol.[11] Once the initial symptoms have passed, a second set of symptoms arises, from 10 to as many as 30 hours after the initial exposure, that may include blurring or complete loss of vision, acidosis, and putaminal hemorrhages, an uncommon but serious complication.[20][25] These symptoms result from the accumulation of toxic levels of formate in the blood, and may progress to death by respiratory failure. Physical examination may show tachypnea, and ophthalmologic examination may show dilated pupils with hyperemia of the optic disc and retinal edema.
Ethanol is sometimes denatured (adulterated), and made poisonous, by the addition of methanol. The result is known as methylated spirit, "meths" (British use) or "metho" (Australian slang). This is not to be confused with "meth", a common abbreviation for methamphetamine and for methadone in Britain.
Applications
Methanol is used primarily as a feedstock for the manufacture of chemicals, and as a fuel for specialized vehicles. As mentioned above, it is a common de-naturing agent. As a common laboratory solvent, is especially useful for HPLC, UV/VIS spectroscopy, and LCMS due to its low UV cutoff.
Chemical industry
Methanol is primarily used in making other chemicals. About 40% of methanol is converted to formaldehyde, and from there into products as diverse as plastics, plywood, paints, explosives, and permanent press textiles.
In the early 1970s, a process was developed by Mobil for producing gasoline fuel for vehicles. One such industrial facility was built at Motunui in New Zealand in the 1980s. In the 1990s, large amounts of methanol were used in the United States to produce the gasoline additive methyl tert-butyl ether (MTBE). While MTBE is no longer marketed in the U.S., it is still widely used in other parts of the world. Methanol (or less commonly, ethanol) is a component in the transesterification of triglycerides for production of biodiesel.
Other chemical derivatives of methanol include dimethyl ether (DME), which has replaced chlorofluorocarbons as an aerosol spray propellant, and acetic acid. Dimethyl ether can be blended with liquified petroleum gas (LPG) for home heating and cooking, and can be used as a replacement for transportation diesel fuel.
Of high interest to the petrochemical marketplace, methanol is an important ingredient in new and lower-cost methods for producing propylene, which is much in demand. Such methods include Methanol-to-Olefins (MTO), Methanol-to-Propylene (MTO/MTP), Metathesis, Propane Dehydrogenation (PDH), High Severity FCC, and Olefins Cracking.
The market for proponyl became tight when the ethane prices fell in the USA with the exploration of shale gas reserves. The low priced ethylene produced from this raw material has given chemical producers in North America a feedstock advantage. Such change has put naphtha-fed steam crackers at a disadvantageous position, with many of them shutting down or revamping to use ethane as feedstock. Nevertheless, the propylene output rates from ethane-fed crackers are negligible.[26]
Fuels of vehicles
Methanol is occasionally used to fuel internal combustion engines. Pure methanol is required by rule to be used in Champcars, Monster Trucks, USAC sprint cars (as well as midgets, modifieds, etc.), and other dirt track series, such as World of Outlaws, and Motorcycle Speedway, mainly because, in the event of an accident, methanol does not produce an opaque cloud of smoke. Since the late 1940s, Methanol is also used as the primary fuel ingredient in the powerplants for radio control, control line, free flight airplanes, cars and trucks; such engines use a platinum filament glow plug that ignites the methanol vapor through a catalytic reaction. Drag racers, mud racers, and heavily modified tractor pullers also use methanol as the primary fuel source. Methanol is required with a supercharged engine in a Top Alcohol Dragster and, until the end of the 2006 season, all vehicles in the Indianapolis 500 had to run on methanol. As a fuel for mud racers, methanol mixed with gasoline and nitrous oxide produces more power than gasoline and nitrous oxide alone.
One problem with high concentrations of methanol in fuel is that alcohols corrode some metals, particularly aluminium. An acid, albeit weak, methanol attacks the oxide coating that normally protects the aluminum from corrosion:
- 6 CH3OH + Al2O3 → 2 Al(OCH3)3 + 3 H2O
The resulting methoxide salts are soluble in methanol, resulting in a clean aluminium surface, which is readily oxidized by dissolved oxygen. Also, the methanol can act as an oxidizer:
- 6 CH3OH + 2 Al → 2 Al(OCH3)3 + 3 H2
This reciprocal process effectively fuels corrosion until either the metal is eaten away or the concentration of CH3OH is negligible. Methanol's corrosivity has been addressed with methanol-compatible materials and fuel additives that serve as corrosion inhibitors.
Organic methanol, produced from wood or other organic materials (bioalcohol), has been suggested as a renewable alternative to petroleum-based hydrocarbons. Low levels of methanol can be used in existing vehicles with the addition of cosolvents and corrosion inhibitors.
Methanol fuel has been proposed for ground transportation. The chief advantage of a methanol economy is that it could be adapted to gasoline internal combustion engines with minimum modification to the engines and to the infrastructure that delivers and stores liquid fuel.
Government policy
The European Fuel Quality Directive allows up to 3% methanol with an equal amount of cosolvent to be blended with gasoline sold in Europe. China uses more than one billion gallons of methanol per year as a transportation fuel in low level blends for conventional vehicles and high level blends in vehicles designed for methanol fuels.
In the US, the Open Fuel Standard Act of 2011 was introduced in the US Congress to encourage car manufacturers to build cars capable of using methanol, gasoline, or ethanol fuels. The bill is being championed by the Open Fuel Standard Coalition.
Other applications
Methanol is a traditional denaturant for ethanol, the product being known as "denatured alcohol" or "methylated spirit". This was commonly used during the Prohibition to discourage consumption of bootlegged liquor, and ended up causing several deaths.[27]
Methanol is used as a solvent and as an antifreeze in pipelines and windshield washer fluid.
In some wastewater treatment plants, a small amount of methanol is added to wastewaterto provide a carbon food source for the denitrifying bacteria, which convert nitrates to nitrogen gas and reduce the nitrification of sensitive aquifers.
During World War II, methanol was used as a fuel in several German military rocket designs, under the name M-Stoff, and in a roughly 50/50 mixture with hydrazine, known as C-Stoff.
Methanol was used as an automobile coolant antifreeze in the early 1900s.[28]
Methanol is used as a destaining agent in polyacrylamide gel electrophoresis.
Direct-methanol fuel cells are unique in their low temperature, atmospheric pressure operation, allowing them to be miniaturized to an unprecedented degree.[29][30] This, combined with the relatively easy and safe storage and handling of methanol, may open the possibility of fuel cell-powered consumer electronics, such as laptop computers and mobile phones.[31]
Methanol is also a widely used fuel in camping and boating stoves. Methanol burns well in an unpressurized burner, so alcohol stoves are often very simple, sometimes little more than a cup to hold fuel. This lack of complexity makes them a favorite of hikers who spend extended time in the wilderness. Similarly, the alcohol can be gelled to reduce risk of leaking or spilling, as with the brand "Sterno".
Methanol is mixed with water and injected into high performance diesel and gasoline engines for an increase of power and a decrease in intake air temperature in a process known as water methanol injection.
Energy carrier
Methanol is useful as an energy carrier because it is easier to store than hydrogen and burns cleaner than fossil fuels.
Methanol is readily biodegradable in both aerobic (oxygen present) and anaerobic (oxygen absent) environments. Methanol will not persist in the environment. The half-life for methanol in groundwater is just one to seven days, while many common gasoline components have half-lives in the hundreds of days (such as benzene at 10–730 days). Since methanol is miscible with water and biodegradable, it is unlikely to accumulate in groundwater, surface water, air or soil.[32]
Safety in automotive fuels
Pure methanol has been used in open wheel auto racing since the mid-1960s. Unlike petroleum fires, methanol fires can be extinguished with plain water. A methanol-based fire burns invisibly, unlike gasoline, which burns with a visible flame. If a fire occurs on the track, there is no flame or smoke to obstruct the view of fast approaching drivers, but this can also delay visual detection of the fire and the initiation of fire suppression. The decision to permanently switch to methanol in American IndyCar racing was a result of the devastating crash and explosion at the 1964 Indianapolis 500, which killed drivers Eddie Sachs and Dave MacDonald.[33] In 2007 IndyCars switched from methanol to ethanol.[34]
Production
From synthesis gas
Carbon monoxide and hydrogen react over a catalyst to produce methanol. Today, the most widely used catalyst is a mixture of copper and zinc oxides, supported on alumina, as first used by ICI in 1966. At 5–10 MPa (50–100 atm) and 250 °C (482 °F), the reaction is characterized by high selectivity (>99.8%):
- CO + 2 H2 → CH3OH
Since the production of synthesis gas from methane produces three moles of hydrogen for every mole of carbon monoxide, whereas the synthesis consumes only two moles of hydrogen gas per mole of carbon monoxide. One way of dealing with the excess hydrogen is to inject carbon dioxide into the methanol synthesis reactor, where it, too, reacts to form methanol according to the equation:
- CO2 + 3 H2 → CH3OH + H2O
In terms of mechanism, the process occurs via iniatial conversion of CO into CO2, which is then hydrogenated:[35]
- CO2 + 3 H2 → CH3OH + H2O
where the H2O byproduct is recycled via the water-gas shift reaction
- CO + H2O → CO2 + H2,
This gives an overall reaction, which is the same as listed above.
- CO + 2 H2 → CH3OH
Other
The catalytic conversion of methane to methanol has long been sought as a route to methanol. This route is effected by enzymes such as methane monooxygenases but commercial routes remain elusive because of the tendency for over-oxidation, i.e., methanol is more readily oxidized than is methane.[36][37]
Feedstocks
Production of synthesis gas
Originally, synthesis gas for the production of methanol came from coal. Today, synthesis gas is most commonly produced from the methane component in natural gas, because natural gas contains hydrogen. Three processes are commercially practiced. At moderate pressures of 4 MPa (40 atm) and high temperatures (around 850 °C), methane reacts with steam on a nickel catalyst to produce syngas according to the chemical equation:
- CH4 + H2O → CO + 3 H2
This reaction, commonly called steam-methane reforming or SMR, is endothermic, and the heat transfer limitations place limits on the size of and pressure in the catalytic reactors used. Methane can also undergo partial oxidation with molecular oxygen to produce syngas, as the following equation shows:
- 2 CH4 + O2 → 2 CO + 4 H2
This reaction is exothermic, and the heat given off can be used in-situ to drive the steam-methane reforming reaction. When the two processes are combined, it is referred to as autothermal reforming. The high pressures and high temperatures needed for steam-reforming require a greater capital investment in equipment than is needed for a simple partial-oxidation process; however, the energy-efficiency of steam-reforming is higher than for partial-oxidation, unless the waste-heat from partial-oxidation is used.
Stoichiometry adjustment
Stoichiometry for methanol production requires the ratio of H2 / CO to equal 2. The partial oxidation process yields a ratio of 2, and the steam reforming process yields a ratio of 3. The H2 / CO ratio can be lowered to some extent by the reverse water-gas shift reaction,
- CO2 + H2 → CO + H2O,
to provide the appropriate stoichiometry for methanol synthesis.
Alternate feedstock materials
Although natural gas is the most economical and widely used feedstock for methanol production, many other feedstocks can be used to produce syngas via steam reforming.[38]Steam-reformed coal is sometimes used as a feedstock for methanol production, particularly in China. In addition, mature technologies available for biomass gasification are being used for methanol production. For instance, woody biomass can be gasified to water gas (a hydrogen-rich syngas), by introducing a blast of steam in a blast furnace. The water-gas / syngas can then be synthesized to methanol using standard methods. The net process is carbon neutral, since the CO2 byproduct is required to produce biomass via photosynthesis. Using a composition for wood of 50% carbon, 42% oxygen, 6% hydrogen[39] we can represent wood with the formula C11H16O7 (we could also use C8H12O5). Then some combination of the following two formal reactions will occur:
- 3 C11H16O7 + 22 H2O → 46 H2 + 23 CO + 10 CO2 → 23 CH3OH + 10 CO2
- 2 C11H16O7 + 11 O2 → 16 H2 + 8 CO + 14 CO2 → 8 CH3OH + 14 CO2
Quality specifications and analysis
Methanol for laboratory use
Methanol is available commercially in various purity grades for fine chemicals:
- "Synthesis" quality (corresponding to normal commercial methanol)
- Certified analytical quality
- Extremely pure qualities for semiconductor manufacture
In addition to laboratory grades, commercial methanol is generally classified according to ASTM purity grades A and AA. Methanol for chemical use normally corresponds to Grade AA. In addition to water, typical impurities include acetone and ethanol (which are very difficult to separate by distillation). When methanol is delivered by ships or tankers used to transport other substances, contamination by the previous cargo must be expected. Comparative ultraviolet spectroscopy has proved a convenient, quick test method for deciding whether a batch can be accepted and loaded. Traces of all chemicals derived from aromatic parent substances, as well as a large number of other compounds, can be detected. Further tests for establishing the quality of methanol include measurements of boiling point range, density, permanganate number, turbidity, color index, and acid number. More comprehensive tests include water determination according to the Karl Fischer method and gas chromatographic determination of byproducts. However, the latter is relatively expensive and time-consuming because several injections using different columns and detectors must be made due to the variety of byproducts present.
History
In their embalming process, the ancient Egyptians used a mixture of substances, including methanol, which they obtained from the pyrolysis of wood. Pure methanol, however, was first isolated in 1661 by Robert Boyle, when he produced it via the distillation of buxus(boxwood).[40] It later became known as "pyroxylic spirit". In 1834, the French chemists Jean-Baptiste Dumas and Eugene Peligot determined its elemental composition.[41]
They also introduced the word "methylene" to organic chemistry, forming it from Greekmethy = "intoxication" + hȳlē = wood (patch of trees), with Greek language errors: "wood (substance)" (Greek ξύλον, xylon) was intended, and the components are in the wrong order for Greek. The term "methyl" was derived in about 1840 by back-formation from "methylene", and was then applied to describe "methyl alcohol". This was shortened to "methanol" in 1892 by the International Conference on Chemical Nomenclature.[42] The suffix -yl used in organic chemistry to form names of carbon groups, was extracted from the word "methyl".
In 1923, the German chemists Alwin Mittasch and Mathias Pier, working for Badische-Anilin & Soda-Fabrik(BASF), developed a means to convert synthesis gas (a mixture of carbon monoxide, carbon dioxide, and hydrogen) into methanol. US patent 1,569,775 was applied for on 4 Sep 1924 and issued on 12 January 1926; the process used a chromium and manganese oxide catalyst with extremely vigorous conditions—pressures ranging from 50 to 220 atm, and temperatures up to 450 °C. Modern methanol production has been made more efficient through use of catalysts (commonly copper) capable of operating at lower pressures. The modern low pressure methanol (LPM) was developed by ICI in the late 1960s US 3326956 with the technology now owned by Johnson Matthey, which is a leading licensor of methanol technology.
Methanol is one of the most heavily traded chemical commodities in the world, with an estimated global demand of around 27 to 29 million metric tons. In recent years, production capacity has expanded considerably, with new plants coming on-stream in South America, China and the Middle East, the latter based on access to abundant supplies of methane gas. Even though nameplate production capacity (coal-based) in China has grown significantly, operating rates are estimated to be as low as 50 to 60%. No new production capacity is scheduled to come on-stream until 2015.
The main applications for methanol are the production of formaldehyde (used in construction and wooden boarding), acetic acid (basis for a.o. PET-bottles), MTBE (fuel component and replacement for the very volatile diethyl ether) and more recently for the formation of methyl esters in the production of bio-diesel. In China, demand is expected to grow exponentially, not only caused by a growing internal market of the traditional applications, but accelerated by new applications, such as direct blending (with gasoline), Methanol-To-Olefins (e.g. propylene) and DME. Methanol can also be used to produce gasoline.
The use of methanol as a motor fuel received attention during the oil crises of the 1970s due to its availability, low cost, and environmental benefits. By the mid-1990s, over 20,000 methanol "flexible fuel vehicles" capable of operating on methanol or gasoline were introduced in the U.S. In addition, low levels of methanol were blended in gasoline fuels sold in Europe during much of the 1980s and early-1990s. Automakers stopped building methanol FFVs by the late-1990s, switching their attention to ethanol-fueled vehicles. While the methanol FFV program was a technical success, rising methanol pricing in the mid- to late-1990s during a period of slumping gasoline pump prices diminished the interest in methanol fuels.[43]
In 2006, astronomers using the MERLIN array of radio telescopes at Jodrell Bank Observatory discovered a large cloud of methanol in space, 288 billion miles across.[44][45]
References
- ^ "Methanol". The PubChemProject. USA: National Center for Biotechnology Information.
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Ballinger, P.; Long, F.A. (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds". J. Am. Chem. Soc. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ^ Refractive index of Methanol, CH3OH (MeOH) [LIQUIDS] – RefractiveIndex.INFO
- ^ González, Begoña (2007). "Density, dynamic viscosity, and derived properties of binary mixtures of methanol or ethanol with water, ethyl acetate, and methyl acetate at T = (293.15, 298.15, and 303.15) K". The Journal of Chemical Thermodynamics 39 (12): 1578–1588. doi:10.1016/j.jct.2007.05.004.
- ^ a b c d "Methanol" (PDF). Lab Chem. Valtech. Retrieved 10 March 2016.
- ^ http://www.methanol.org/Health-And-Safety/Safety-Resources/Health---Safety/Methanex-TISH-Guide.aspx
- ^ a b c d "NIOSH Pocket Guide to Chemical Hazards #0397". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c "Methanol". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ^ "The Emergency Response Safety and Health Database: Systematic Agent: METHANOL". Centers for Disease Control and Prevention. Retrieved 26 August 2009.
- ^ a b National Institute for Occupational Safety and Health (22 August 2008). "The Emergency Response Safety and Health Database: Methanol". Retrieved 17 March 2009.
- ^ Brooks Hays (17 April 2015). "Why astronomers hate the lawn-mowing Roomba". Space Daily.
- ^ Barceloux, D. G.; Bond, G. R.; Krenzelok, E. P.; Cooper, H; Vale, J. A.; American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning (2002). "American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning". Journal of toxicology. Clinical toxicology 40 (4): 415–46. PMID 12216995.
- ^ Turner, C; Spanel, P; Smith, D (2006). "A longitudinal study of methanol in the exhaled breath of 30 healthy volunteers using selected ion flow tube mass spectrometry, SIFT-MS". Physiological Measurement 27 (7): 637–48. doi:10.1088/0967-3334/27/7/007. PMID 16705261.
- ^ Lindinger, W; Taucher, J; Jordan, A; Hansel, A; Vogel, W (1997). "Endogenous production of methanol after the consumption of fruit". Alcoholism: Clinical and Experimental Research 21 (5): 939–43. doi:10.1111/j.1530-0277.1997.tb03862.x. PMID 9267548.
- ^ a b Vale A (2007). "Methanol". Medicine 35 (12): 633–4. doi:10.1016/j.mpmed.2007.09.014.
- ^ "Methanol Poisoning Overview". Antizol. Archived from the original on 5 October 2011.
- ^ Methanol (CASRN 67-56-1)
- ^ Blum, Deborah (2011). The Poisoner's Handbook. Penguin Books. p. 231. ISBN 014311882X.
- ^ a b c d e f Schep LJ, Slaughter RJ, Vale JA, Beasley DM (2009). "A seaman with blindness and confusion". BMJ 339: b3929. doi:10.1136/bmj.b3929. PMID 19793790.
- ^ McMartin KE, Martin-Amat G, Noker PE, Tephly TR (1979). "Lack of a role for formaldehyde in methanol poisoning in the monkey". Biochem. Pharmacol. 28 (5): 645–9. doi:10.1016/0006-2952(79)90149-7. PMID 109089.
- ^ Liesivuori J, Savolainen H (September 1991). "Methanol and formic acid toxicity: biochemical mechanisms". Pharmacol. Toxicol. 69 (3): 157–63. doi:10.1111/j.1600-0773.1991.tb01290.x. PMID 1665561.
- ^ Casavant MJ (Jan 2001). "Fomepizole in the treatment of poisoning". Pediatrics 107(1): 170–171. doi:10.1542/peds.107.1.170. PMID 11134450.
- ^ Brent J (May 2009). "Fomepizole for ethylene glycol and methanol poisoning". N Engl J Med 360 (21): 2216–23. doi:10.1056/NEJMct0806112. PMID 19458366.
- ^ Permpalung N, Cheungpasitporn W, Chongnarungsin D, Hodgdon TM (Oct 2013). "Bilateral putaminal hemorrhages: serious complication of methanol intoxication". N Am J Med Sci 5 (10): 623–4. doi:10.4103/1947-2714.120804. PMC 3842708. PMID 24350079.
- ^ "Propylene Production from Methanol". by Intratec, ISBN 978-0-615-64811-8.
- ^ Blum, Deborah (19 February 2010). "The little-told story of how the U.S. government poisoned alcohol during Prohibition". Slate Magazine. Retrieved 10 June 2010.
- ^ Yant, W. P.; Schrenk, H. H.; Sayers, R. R. (1931). "Methanol Antifreeze and Methanol Poisoning". Industrial & Engineering Chemistry 23 (5): 551. doi:10.1021/ie50257a020.
- ^ Kamitani, A.; Morishita, S.; Kotaki, H.; Arscott, S. (2008). "Miniaturized microDMFC using silicon microsystems techniques: Performances at low fuel flow rates". Journal of Micromechanics and Microengineering 18 (12): 125019. doi:10.1088/0960-1317/18/12/125019.
- ^ Kamitani, A.; Morishita, S.; Kotaki, H.; Arscott, S. (2011). "Microfabricated microfluidic fuel cells". Sensors and Actuators B: Chemical 154 (2): 174. doi:10.1016/j.snb.2009.11.014.
- ^ Berger, Sandy (30 September 2006). "Methanol Laptop Fuel". Compu·Kiss. Retrieved 22 May 2007.
- ^ Evaluation of the Fate and Transport of Methanol in the Environment, Malcolm Pirnie, Inc., January 1999.
- ^ McDonald, Norris (21 April 2007). "Green no longer bad luck at Indy". Toronto Star. Retrieved 12 May 2010.
- ^ "IndyCar Series Teams Begin Use of Ethanol-Blended Fuel". Indycar.com. 1 December 2005. Retrieved 7 November 2010.
- ^ Olaf Deutschmann, Helmut Knözinger, Karl Kochloefl, Thomas Turek "Heterogeneous Catalysis and Solid Catalysts, 3. Industrial Applications" in Ullmann's Encyclopedia of Industrial Chemistry" 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o05_o03
- ^ Alayon, E. M. C.; Nachtegaal, M.; Ranocchiari, M.; Van Bokhoven, J. A. (2012). "Catalytic Conversion of Methane to Methanol Using Cu-Zeolites". CHIMIA International Journal for Chemistry 66 (9): 668–674. doi:10.2533/chimia.2012.668. PMID 23211724.
- ^ Hammond, C.; Jenkins, R. L.; Dimitratos, N.; Lopez-Sanchez, J. A.; Ab Rahim, M. H.; Forde, M. M.; Thetford, A.; Murphy, D. M.; Hagen, H.; Stangland, E. E.; Moulijn, J. M.; Taylor, S. H.; Willock, D. J.; Hutchings, G. J. (2012). "Catalytic and Mechanistic Insights of the Low-Temperature Selective Oxidation of Methane over Cu-Promoted Fe-ZSM-5". Chemistry - A European Journal 18 (49): 15735. doi:10.1002/chem.201202802. PMID 23150452.
- ^ Methanol Basics. afdc.energy.gov
- ^ Barette, Jean-Pierre; Hazard, Claude; Mayer, Jérôme (1996). Mémotech Bois et Matériaux Associés. Paris: Éditions Casteilla. p. 22. ISBN 27135-1645-5.
- ^ Boyle discusses the distillation of liquids from the wood of the box shrub in: Robert Boyle, The Sceptical Chymist (London, England: J. Cadwell, 1661), pp. 192–195.
- ^ A report on methanol to the French Academy of Sciences by J. Dumas and E. Péligot began during the Academy's meeting of October 27, 1834 and finished during the meeting of November 3, 1834. See: Procès-verbaux des séances de l'Académie, 10 : 600–601. Available on: Gallica. The complete report appears in: J. Dumas and E. Péligot (1835) "Mémoire sur l'espirit de bois et sur les divers composés ethérés qui en proviennent"(Memoir on spirit of wood and on the various ethereal compounds that derive therefrom), Annales de chimie et de physique, 58 : 5–74; from page 9: Nous donnerons le nom deméthylène (1) à un radical … (1) Μεθυ, vin, et υλη, bois; c'est-à-dire vin ou liqueur spiritueuse du bois. (We will give the name "methylene" (1) to a radical … (1) methy, wine, and hulē, wood; that is, wine or spirit of wood.)
- ^ For a report on the International Conference on Chemical Nomenclature that was held in April 1892 in Geneva, Switzerland, see:
- Lockyer, Sir Norman; Lockyer, J; Kirby, F; Sargeant, J; Fleet, L; Wright, D (1892). "Nature". Nature 46 (1177): 240–5. Bibcode:1892Natur..46...56A. doi:10.1038/046056c0. PMID 16042519.
- Armstrong's report is reprinted with the resolutions in English in: Armstrong, Henry (1892). "The International Conference on Chemical Nomenclature". The Journal of Analytical and Applied Chemistry 6: 390–400.
p. 398: 15. The alcohols and the phenols will be called after the name of the hydrocarbon from which they are derived, terminated with the suffix ol (ex. pentanol, pentenol, etc.).
- ^ Halderman, James D.; Martin, Tony (2009). Hybrid and alternative fuel vehicles. Pearson/Prentice Hall. ISBN 978-0-13-504414-8.
- ^ "Upgraded MERLIN spies cloud of alcohol spanning 288 billion miles" (Press release). Jodrell Bank Centre for Astrophysics. 19 April 2006.
- ^ Amos, Jonathan (5 April 2006). "Merlin sees vast alcohol stream". BBC News.
Further Reading
- Robert Boyle, The Sceptical Chymist (1661) – contains account of distillation of wood alcohol.
External Links
- International Chemical Safety Card 0057
- The Methanol Institute Industry trade group, lots of information on methanol's use in fuel cells and as an alternative fuel.
- Methyl Alcohol (Methanol) CDC/NIOSH, links to safety information
- CDC – NIOSH Pocket Guide to Chemical Hazards – Methyl Alcohol
- China Takes Gold in Methanol Fuel
- The methanol story: a sustainable fuel for the future article by Ford Motor's Roberta Nichols, the mother of the flexible fuel vehicle, discussing Gasoline-Ethanol-Methanol flexibility in the Journal of Scientific & Industrial Research
- Methanol Fact Sheet – National Pollutant Inventory
- Methanol Discovered in Space
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of methanol
Wikipedia












No comments:
Post a Comment