Title
ABSTRACT: Thermoplastic starch (TPS) was obtained from natural and acetylated cassava starch using a twin screw extruder and then conditioned at 25 ºC and 54 % of relative humidity. It was found that the crystallinity index, calculated as the ratio of the IR peaks at 1047 (crystalline phase) and 1022 cm-1 (amorphous phase), decreases due to the effect of a plasticization process. Also, as expected, SEM micrographs show that the plasticization process destroyed the starch granular structure almost completely and an amorphous mass was obtained. The TGA results indicated that the activation energy, Ea, was also reduced by the plasticization process. The acetylated TPS shows a decrease in Tg, in tensile strength and in the percentage of moisture absorption compared to natural TPS but a larger strain at the breaking point. This behavior suggests that the chemical modification reduces the secondary interactions between starch chains due to the substitution of the hydroxyl groups by acetates.
KEYWORDS: Thermoplastic starch, cassava starch, acetylated starch, biodegradable materials, starch plasticization, renewable materials.
RESUMEN: Almidón de Yuca natural y acetilado fueron plastificados empleando un extrusor de doble husillo y almacenados a 25 °C y 54 % de humedad relativa. El índice de cristalinidad, estimado como el cociente de las bandas de FTIR a 1047 (fase cristalina) y a 1022 cm-1 (fase amorfa) disminuyó en ambos almidones debido al proceso de plastificación. Mediante las micrografías de SEM se pudo observar que el proceso de plastificación destruyó casi por completo la estructura granular de los almidones. Los resultados de TGA mostraron que la energía de activación, Ea, disminuyó con el proceso de plastificación. Por otra parte se encontró que el almidón termoplástico acetilado presentó una Tg, absorción de agua y resistencia a tensión menores en comparación con el almidón termoplástico natural, mientras que su elongación a la ruptura fue mayor. Este comportamiento sugirió que la esterificación de los grupos hidroxilos del almidón natural redujeron las interacciones entre las cadenas del almidón plastificado.
PALABRAS CLAVE: Almidón termoplástico, almidón de yuca, almidón acetilado, materiales biodegradables, plastificación de almidón, materiales renovables.
1. INTRODUCTION
Recent research efforts in the area of polymeric materials have focused on the development of biodegradable materials obtained from renewable resources such as starch, proteins, hydroxyalkanoates, etc., which are liable to complete biodegradability when subjected to composting conditions [1, 2, 3]. Nowadays, due to the worldwide decrease of oil production, the search for new materials from renewable resources becomes crucial for substituting traditional synthetic polymers. Among these materials stands out thermoplastic starch (TPS) since the starches are the most abundant and cheapest materials that come from renewable resources [1, 4].
Starch consists of two major components: amylose, a mostly linear α-D(1,4)- glucan and amylopectin, an α-D-(1-4)-glucan which has α-D(1,6) linkages at the branch point. The linear amylose molecules of starch have a molecular weight of 0.2–2 million, whereas the branched amylopectin molecules have molecular weights as high as 100–400 million [5]. Most often the structure of starch is chemically modified in order to decrease the number of hydroxyl groups in the search for a number of possible applications. Thereby, starch has been treated by hydroxipropylation [6], oxidation [7] and acrylic grafting, mainly. However, in recent years, there has been a considerable research effort with acetylated thermoplastic starch, because of the experience acquired at food applications where acetylation is the second most studied treatment after crosslinking [8]. Acetylation is the esterification of starch with acetic anhydride, used to facilitate the formation of acetates groups, which has been performed mainly to adjust the viscosity of food and to improve its stability and its resistance to degradation as compared to untreated starch. Also, the incorporation of acetate groups reduces the interaction between chains and improves the swelling power and the solubility of starch granules. The change of the physicochemical properties of starch is proportional to the substitution degree of the formed acetate groups [9].
In order to change a native starch into a bioplastic material it is necessary to break up its semicrystalline granular structure [10]. A starch without the appropriate additives (plasticizers) does not possess the necessary properties to perform as a thermoplastic. The plasticizers increase the flexibility of starch because of their ability to reduce the formation of hydrogen bonds and increase molecular separation [11]. In the field of food science, water is considered to be the best plasticizer. However, in the case of TPS, water is included as an aid to the main additive, glycerol being the most studied. In order to improve the poor mechanical properties of starch and to reduce its water absorption, several studies found in the literature deal with different aspects concerning the improvement of plasticizers [2, 12], the more stable biodegradable polymer blends [13, 14], the incorporation of natural fibers [15, 16] and as mentioned before, the chemical modifications by acetylation is the most studied. It is worth mentioning that most of these studies were performed on starches obtained from corn, barley, potato, and rice.
The purpose of this paper is to characterize two biodegradable types of thermoplastic starch obtained from natural and acetylated cassava starch (Manihot sculenta crantz) by their physicochemical, thermal and mechanical properties.
2. EXPERIMENTAL METHODOLOGY
2.1 Materials Cassava starch samples, natural food grade and acetylated industrial grade, were kindly provided by Industrias del Maíz S.A. (Corn Products Andina Colombia), from Cali, Colombia and were used as received. The glycerol used was industrial grade.
2.2 Preparation of thermoplastic starch
Similar to the process reported by Huang [3] and Ma [17], 2006, both the cassava starch and the acetylated one were first dried for 24 hours at 80°C and then mixed with glycerol in a 70:30 weight ratio using a Black and Decker high speed mixer, until the lumps disappeared (around five minutes). The mixture was then stored in a sealed container for 72 hours. Finally, it was plasticized using a co-rotating twin screw extruder coupled to a Brabender plasticorder Model PLE 330, equipped with a 32 mm conical cylinder and an L/D ratio of 13. The rotating speed was kept at 45 rpm and the temperature profile used was 115, 125, 130 and 135 ºC for all three zones of the screw and the head respectively.
2.3 Fourier Transform Infrared Spectroscopy (FTIR) Fourier Transform Infrared Spectroscopy analysis was performed on a Nicolet model Protégé 460 magna IR spectrometer. The analysis of the milled starch (natural and acetylated) was carried out with analytical grade KBr pellets using the transmission mode. The spectra were recorded with 4 cm-1 resolution and 100 scans. In the case of the TPS, a microscope with an accessory for attenuated total internal reflectance (ATR) spectroscopy was used.
2.4 Thermogravimetric analysis (TGA)
Thermal stability was evaluated using a Perkin Elmer TGA 7 in a temperature range between 50 and 650 °C, with a heating rate of 10 ºC/min and an inert atmosphere, nitrogen gas flow of 20 ml/min. The average weight of the evaluated samples was 7 mg.
2.5 Scanning electron microscopy (SEM)
The morphology of the milled starch particles and the failure surfaces of both the natural and acetylated thermoplastic starches were observed using a scanning electron microscope JEOL SEM Model LV 5400. The samples were coated with gold prior to the analysis.
2.6 Mechanical properties Tensile mechanical properties of both, the natural and acetylated thermoplastic starches were determined after the room conditioning of the samples. The testing was carried out only when the hygro-thermal equilibrium had been achieved at 54 % RH and a temperature of 25 ºC. The tensile testing was performed using a Shimadzu Model AG-1 100 KN universal testing machine equipped with a 500 N load cell. Type IV samples were prepared according to the ASTM D-638 standard specifications using a cross-head speed of 5 mm/min.
2.7 Moisture absorption Isotherms The thermoplastic starch samples were dried in an oven at 80 °C during 12 hours and then placed in a desiccator with an aqueous solution of hexa hydrated magnesium nitrate in order to maintain a relative humidity of approximately 54 ± 2 %. Weight gain data were obtained as a function of time (Pt) at a temperature of 25 ± 2 ºC, moisture absorption (H) was calculated as a percentage, taking the weight obtained right after oven drying (Ps) as the initial value, according to (1).
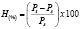 (1)
(1)
2.8 Dynamical mechanical analysis (DMA) A Dynamical Mechanical Analyzer (Perkin Elmer DMA 7) was used for the determination of the second order transitions of both the natural thermoplastic starch and the acetylated thermoplastic starch, using the extension mode. The specimen size was 2 mm x 1 mm x 6 mm. The samples were analyzed at a temperature range between -100 and 60 ºC (cooling with liquid nitrogen), using a heating rate of 5 °C/min, a frequency of 1 Hz and a gas nitrogen flow of 20 ml/min.
3. RESULTS AND DISCUSSION
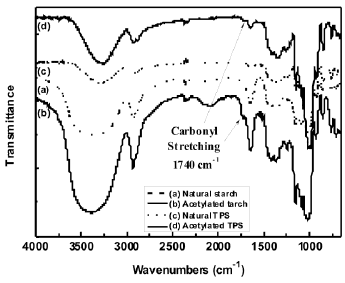
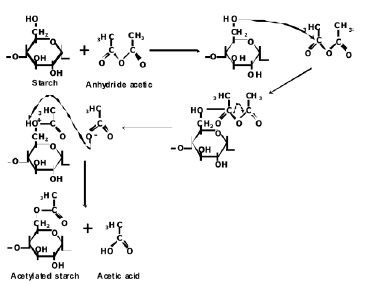
3.1 Fourier transform infrared spectroscopy (FTIR) The FTIR spectra for both the natural and acetylated starches are shown in Figure 1. The assignment of the corresponding bands for both starches is presented in Table 1. The main difference in the spectra of the milled starches is attributed to the appearance of new bands at 1740, 1375 and 1240 cm-1, characteristic of the acetate group formed in the acetylation process. A typical reaction for such a process is illustrated in Figure 2. Here, the structure of the repeat units where the chemical modification is performed is presented. These bands can also be observed in the spectrum corresponding to the acetylated TPS. In Figure 3, the interval from 800 to 1400 cm-1 of the starches studied is shown. Smits et al. [18] pointed out that the band for 1047 cm-1 is related to the crystalline phase of starch, while the band at 1022 1047 cm-1 is related to the amorphous one. From a reference line drawn between 1180 and 880 cm-1, the index of crystallinity was calculated as the band height ratio (H1047/H1022).
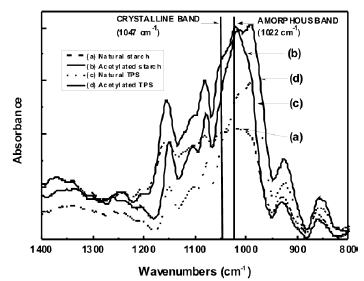
It was found that the crystallinity indexes were 0.911 and 0.821 for the natural and acetylated milled starches respectively, and 0.694 and 0.716 for the natural and acetylated thermoplastic starch. These results indicate, as expected, that the extrusion processes decrease the crystallinity of both types of starch, more noticeable for natural starch compared with the acetylated starch. The effect of the acetylation on the morphology of starches is more complex. On one side, the acetylation, at least at the surface level, partially decreases the arrangement of the starch granules, in agreement with the distortion related to the loss of symmetry upon the replacement of the hydroxyl groups –OH by the acetate groups –CH3OCO, which are larger.
Also, when comparing the crystallinity indexes, between the thermoplastic starches, the acetylation process appears to give place to a highly ordered TPS, when compared to the natural starch. This behavior tends to suggest that the interactions between starch and glycerol play an important role in the plasticization process.
3.2 Thermogravimetric analysis (TGA) The loss of mass occurring along the temperature range selected for this study, revealed that, with the plasticization process of starch, thermal stability is lost, as shown in Figure 4; such a decrease is mainly attributed to the incorporation of glycerol, a low molecular weight molecule, whose evaporation and/or decomposition occurs at lower temperatures than the starches. Figure 5 shows the derivative of the thermogravimetric analysis curves.
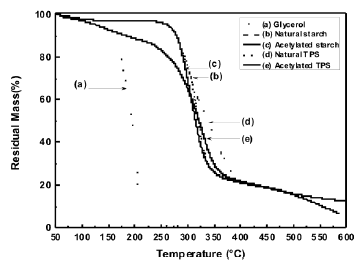
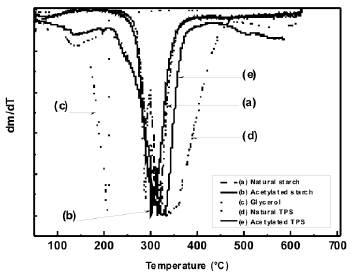
The temperature for the higher rate of mass loss of these materials can clearly be observed. It can also be seen that the plasticized starch presents small shifts to the right for the peak and that the acetylation process induces a shift of the peak to the left for both the plasticized starch and the milled-like granular structure. It is observed that for glycerol there is a peak value at 209 °C, where the largest mass loss occurs, as depicted by an almost vertical drop of the thermogram in Figure 4.
The kinetic parameters for the materials can be calculated from the model proposed by Broido [20], as shown in (2).
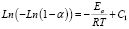 (2)
(2)
Where:
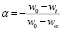 (3)
(3)
The activation energy can be estimated from the calculation of the slope of the curve given by (2); it should be pointed out that, in the graph for Broido’s model there are three different lineal regions in the temperature range which are also associated with three different activation energies.
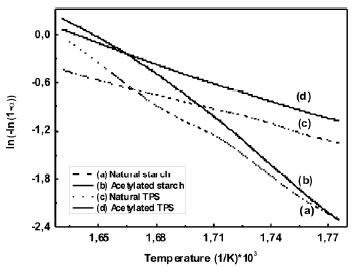
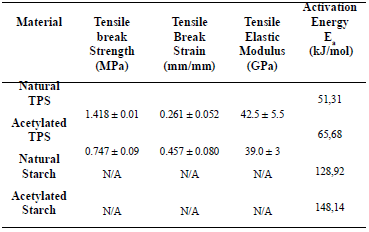
These regions have also been reported by other researchers [18, 21] in studies of dry and plasticized starch. Figure 6 shows the values of the activation energy for the central temperature region (290 – 340 ºC), which are shown in Table 2 where the peak value of the curve for the derivative occurs.
The value obtained for the activation energy for natural cassava starch was 129 kJ/mol. A value of 102 kJ/mol was reported by Alvarez and Vazquez [22] for corn starch calculated using the Kissinger method. It should be pointed out that the calculated values for the kinetic parameters vary according to the type
of atmosphere, the shape and amount of the sample; the flow and heat rate; and the method used for the calculations. In the case of the thermoplastic starch, the secondary interactions between the polymeric chains are smaller, compared with the non-plasticized starch, due to the presence of glycerol. Therefore, it is expected that the activation energy for the thermal degradation process of the TPS should be smaller than the one for the milled starch.
The value of Ea for natural TPS, was 51.3 kJ/mol, a value of 63.3 kJ/mol was reported by Valles et al. [21], for a mixture of thermoplastic starch, also plasticized with glycerol. This value was also calculated using Broido’s model for a temperature interval similar to the one used in this study.
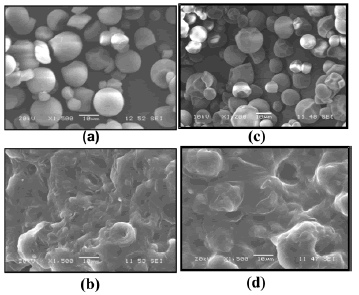
3.3 Scanning electron microscopy Figures 7 (a) and 7 (c) show micro photographs of the morphology for natural and acetylated cassava starch particles, used in the plasticization process, respectively. As stated by Wurzburg [23], the geometry of this type of starches is sphere-like, as opposed to those from other botanical sources which posses a polygonal shape. The presence of the plasticizer and the resulting shear stress led to the breakage of the granular structure of starch. However, as seen in the microphotographs Figures 7(b) and 7(d), there still are a few granules that are not totally fragmented; this is an indication that the processing conditions can still be improved. The last microphotographs were taken from the failure cross-section of tensile test samples.
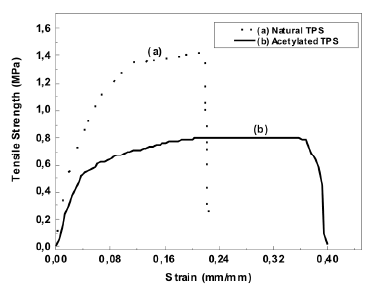
3.4 Mechanical properties The tensile strength found for the natural and the thermoplastic starch are low compared to the 5.5 MPa value reported by Huang et al. [2] for corn starch with similar glycerol contents. It should be pointed out that they used a higher strain rate (10 mm/min) and a lower relative humidity (50 %) than those used in this study (5 mm/min y 54 %). Also, as reported by Mathew [24], for a system of corn starch with similar glycerol contents, the tensile strength, elastic modulus and strain at break were 0.23 MPa, 38 MPa, and 14 %, respectively. Ruiz [25] reported a tensile strength of 0.38 MPa, and strain at breaking point of 69 % for a thermoplastic starch with a 65 % modified cassava starch and 35 % glycerol. It is important to point out that the mechanical properties of the TPS vary with time, and with the temperature and relative humidity they are exposed to [26]. This is attributed to the water plasticization and retrogradation phenomena. These are is the main reasons why there are always differences in the reported values for this type of characterization.
The typical stress-strain curves for both natural and acetylated TPS are shown in Figure 8, and the average values of five specimens tested can be seen in Table 2. Figure 8 shows that the mechanical behavior of both TPSs is different. The acetylated TPS has lower tensile break strength and a higher tensile break strain compared to natural TPS. This finding suggests that the role of glycerol during the plasticization was more effective for the chemically modified starch than for the natural one. In the case of the tensile modulus, no statistical differences were found.
3.5 Isotherms of moisture absorption Figure 9 shows the curves for the isotherms of moisture absorption from the thermoplastic starches. It was found that the moisture absorption for the conditions in this study was of the order of 10 %, and a slight decrease in the absorption was evident for the acetylated material.
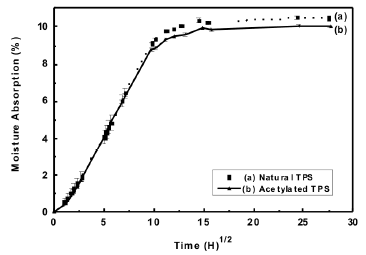
As expected, this decrease of the absorption suggests that there is a decrease in the number of hydroxyl groups, attributed to the acetylation of the starch, thus influencing the capacity for the formation of bonds between the material and water molecules from the environment.
Furthermore, a noticeable decrease in the absorption of moisture could be expected for the starches presenting higher percentages of substitutions. Similar values of moisture absorption have been reported by Curvelo [15] for a starch plasticized with 30 % glycerol; as reported in these studies, an absorption of approximately 9 % is generated for equilibrium conditions when the relative humidity and the temperature are kept at 43 % and 25 ºC respectively.
3.6 Dynamical mechanical analysis Figure 10 shows the curves for Tan d obtained from a DMA for both, the natural and acetylated TPS. As can be seen, there are two second order transitions β and α, which have been amply reported in the literature [27].
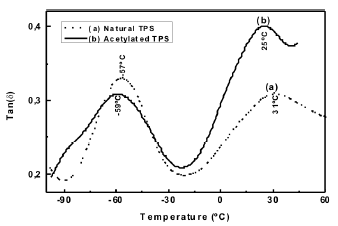
The first peak is associated with local movements attributed to the glycerol molecules, while the second transition is commonly denoted as the glass transition and is related to the movements when the energy is high enough to generate conformational changes at the molecular chain level. In a similar study Da Roz [28] evaluated different plasticizers (ethilenglycol, sorbytol, polyethilenglycol, 1-4 butanodiol, etc.) used as additives for corn starch TPS; he reported transitions in similar intervals to those obtained via DMA and shown in Figure 10. On the other hand, we observe that the chemical modification of starch at low substitution degrees generates a decrease in the associated temperatures related to the transitions, suggesting that, in agreement with the analysis of the moisture absorption isotherms, little effect exist in the decrease of the secondary interactions between the starch molecules, also attributed to the decrease in the number of hydroxyl groups.
4. CONCLUSIONS
The acetylation treatment of cassava starch, contributed to the increase of the amorphous region of the material. This was made evident by the relative decrease of the IR band at 1047 cm-1 associated with the crystalline regions of the material. The water absorption capability of the acetylated starch was smaller than that of the natural one despite the lower acetylation degree. This fact suggests that an increase in the degree of chemical modification could contribute to further diminishing the capability of moisture absorption of the TPS. Although the acetylation process decreases the mechanical strength of the TPS it seems that the better interaction of the glycerol with the modified starch would allow us to decrease it in order to improve the TPS mechanical properties.
5. ACKNOWLEDGEMENTS
This work was performed with the support of the Centro de Investigación Científica de Yucatán A.C. (CICY) México, the Universidad del Valle, COLCIENCIAS and the Centro de Excelencia de Nuevos Materiales (CENM), Colombia. The authors also express their gratitude to Industrias del Maíz for having provided the cassava starch and Q.I. Tanit Toledano-Thompson for the SEM micrographs.
REFERENCES
[1] GUAN, J., ESKRIDGE, K. AND HANNA, M. Acetylated starch-polylactic acid loose-fill packaging materials, Industrial Crops and Products, 22, 109-123, 2004. [ Links ]
[2] HUANG, M., YU, J. AND MA, X. Ethanolamine as a novel plasticizer for thermoplastic starch, Polymer Degradation and Stability, 90, 501-507, 2005. [ Links ]
[3] VILLADA, H. Influencia de mezclas de almidón agrio, perfil de temperatura y velocidad de tornillo de un extrusor sencillo en la producción de almidón termoplástico, su caracterización fisicoquímica, mecánica, microestructural y comportamiento durante el almacenamiento [Tesis Ph.D.]. Universidad del Valle, Facultad de Ingeniería, 2005. [ Links ]
[4] LAI, S., DON, T. AND HUANG, Y. Preparation and Properties of Biodegradable Thermoplastic Starch/Poly(hydroxyl butyrate) Blends, Journal of Applied Polymer Science, 100, 2371-2379, 2006. [ Links ]
[5] HUANG, M., YU, J. AND MA, X. Ethanolamine as a novel plasticizer for thermoplastic starch, Polymer Degradation and Stability, 90, 501-507, 2005. [ Links ]
[6] KIM, D-H., NA, S-K. AND PARK, J-S. Preparation and Characterization of Modified Starch-Based Plastic-Film Reinforced with Short Pulp Fiber. I. Structural Properties, Journal of Applied Polymer Science, 88, 2100-2107, 2003. [ Links ]
[7] ACIOLI, R. The effect of physical aging, Starch particle size, and Starch oxidation on thermal-mechanical properties of Poly(lactic acid)/Starch composites, [Thesis PhD]. College of Agriculture. Kansas State University, 2006.
[8] WANG, X. et al. Study on the morphology, crystalline structure and thermal properties of yam starch acetates with different degrees of substitution, Science in China Series B: Chemistry, 51, 9, 859-865, 2008. [ Links ]
[9] NAVDEEP, S. AND NARPINDER, S. Characteristics of acetylated starches prepared using starches separated from different rice cultivars, Journal of Food Engineering, 70, 117-127, 2005. [ Links ]
[10] THIRE, R., SIMAO, R. AND ANDRADE, C. Investigation of the Surface Morphology of Plasticized Corn starch Films, Act Microscopic, 12, 1, 175-179. 2003. [ Links ]
[11] MALI, L., SAKANAKA, F. AND YAMASHITA, M. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect, Carbohydrate Polymers, 60, 283-289, 2005. [ Links ]
[12] MA, X., YU, G. AND WAN, J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch, Carbohydrate Polymers, 64, 267-273. 2006. [ Links ]
[13] AVEROUS, L., MORO, L., DOLE, P. AND FRAINGANT, C. Properties of thermoplastic blends: starch- polycaprolactone, Polmer, 41, 41574167, 2000. [ Links ]
[14] SARAZIN, P., Ll, G., ORTS, W. AND FAVIS, B. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch, polymer, 49, 599609, 2008. [ Links ]
[15] CURVELO, A., CARVALHO, A. AND AGNELLI, J. Thermoplastic starch-cellulosic fibers composites: preliminary results, Carbohydrate Polymers, 45, 183-188, 2001. [ Links ]
[16] MA, X., YU, J. AND KENNEDY, J. Studies on the properties of natural fibers-reinforced thermoplastic starch composites, Carbohydrate Polymers, 62, 19-24, 2005. [ Links ]
[17] MA, X., YU, G. AND WAN, J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch, Carbohydrate Polymers, 64, 267-273. 2006. [ Links ]
[18] SMITS, A., RUHNAU, F., VLIEGENTHART, J. AND VAN SOEST, J. Ageing of Starch Based System as Observed with FTIR and Solid State NMR Spectroscopy, Starch/Starke, 50, 478483, 1998. [ Links ]
[19] MANO, J.F., KONIAROVA, D. AND REIS, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability, Journal of Materials Science: Materials in Medicine, 14, 127-135, 2003. [ Links ]
[20] BROIDO, A. A Simple, Sensitive Graphical Method of Treating Thermogravimetric Analysis Data, Journal of Polymer Science, A-2, 7, 1761. 1969. [ Links ]
[21] VALLÉS, A. et al. Thermal Analysis Characterization of the Degradation of Biodegradable Starch Blends in Soil, Journal of Applied Polymer Science, 96, 358-371, 2005. [ Links ]
[22] ALVAREZ, V. AND VAZQUEZ, A. Thermal degradation of cellulose derivates/starch blends and sisal fibre biocomposites, Polymer Degradation and Stability, 84, 13-21, 2004. [ Links ]
[23] WURZBURG, O. Modified Starches: Properties and Uses, CRC Press, 2000. [ Links ]
[24] MATHEW, A. AND DUFRESNE, A. Plasticized Waxy Maize Starch: Effect of Polyols and Relative Humidity on Material Properties, Biomacromolecules, 3, 1101-1108. 2002. [ Links ]
[25] RUIZ, G. Obtención y caracterización de un polímero biodegradable a partir del almidón de yuca, Ingeniería y Ciencia, 2, No. 4, 5-28. 2006. [ Links ]
[26] MINA, J., VALADEZ, A., HERRERA-FRANCO, P. AND TOLEDANO, T. Influencia del tiempo de almacenamiento en las propiedades estructurales de un almidón termoplástico de yuca (TPS), Ingeniería y Competitividad, 11, No. 2, 95-106. 2009. [ Links ]
[27] LOURDIN, D., BIZOT, H. AND COLONNA, P. Antiplasticization in Starch-Glycerol Films?, Journal of Applied Polymer Science, 63, 8, 1047-1053, 1997. [ Links ]
[28] DA, R. The effect of plasticizers on thermoplastic starch compositions obtained by melt processing, Carbohydrate Polymers, 63, 417-424, 2006. [ Links ]
For further details log on website :
http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0012-73532011000200020
PHYSICOCHEMICAL CHARACTERIZATION OF NATURAL AND ACETYLATED THERMOPLASTIC CASSAVA STARCH
CARACTERIZACIÓN FISICOQUÍMICA DE ALMIDÓN TERMOPLÁSTICO DE YUCA NATURAL Y ACETILADA
JOSE MINA
Escuela de Ingeniería de Materiales, Universidad del Valle, Cali (Colombia), johermin@univalle.edu.co
ALEX VALADEZ-GONZÁLEZ
Unidad de Materiales, Centro de Investigación Científica de Yucatán A.C. (CICY), Mérida (México), avaladez@cicy.mx
PEDRO HERRERA-FRANCO
Unidad de Materiales, Centro de Investigación Científica de Yucatán A.C. (CICY), Mérida (México), pherrera@cicy.mx
FABIO ZULUAGA
Grupo de síntesis y mecanismos en química orgánica,Universidad del Valle, Cali (Colombia), fazulu@univalle.edu.co
SILVIO DELVASTO
Escuela de Ingeniería de Materiales, Universidad del Valle, Cali (Colombia), delvasto@univalle.edu.co
Received for review November 27th, 2009, accepted April 22th, 2010, final version April, 30th, 2010
ABSTRACT: Thermoplastic starch (TPS) was obtained from natural and acetylated cassava starch using a twin screw extruder and then conditioned at 25 ºC and 54 % of relative humidity. It was found that the crystallinity index, calculated as the ratio of the IR peaks at 1047 (crystalline phase) and 1022 cm-1 (amorphous phase), decreases due to the effect of a plasticization process. Also, as expected, SEM micrographs show that the plasticization process destroyed the starch granular structure almost completely and an amorphous mass was obtained. The TGA results indicated that the activation energy, Ea, was also reduced by the plasticization process. The acetylated TPS shows a decrease in Tg, in tensile strength and in the percentage of moisture absorption compared to natural TPS but a larger strain at the breaking point. This behavior suggests that the chemical modification reduces the secondary interactions between starch chains due to the substitution of the hydroxyl groups by acetates.
KEYWORDS: Thermoplastic starch, cassava starch, acetylated starch, biodegradable materials, starch plasticization, renewable materials.
RESUMEN: Almidón de Yuca natural y acetilado fueron plastificados empleando un extrusor de doble husillo y almacenados a 25 °C y 54 % de humedad relativa. El índice de cristalinidad, estimado como el cociente de las bandas de FTIR a 1047 (fase cristalina) y a 1022 cm-1 (fase amorfa) disminuyó en ambos almidones debido al proceso de plastificación. Mediante las micrografías de SEM se pudo observar que el proceso de plastificación destruyó casi por completo la estructura granular de los almidones. Los resultados de TGA mostraron que la energía de activación, Ea, disminuyó con el proceso de plastificación. Por otra parte se encontró que el almidón termoplástico acetilado presentó una Tg, absorción de agua y resistencia a tensión menores en comparación con el almidón termoplástico natural, mientras que su elongación a la ruptura fue mayor. Este comportamiento sugirió que la esterificación de los grupos hidroxilos del almidón natural redujeron las interacciones entre las cadenas del almidón plastificado.
PALABRAS CLAVE: Almidón termoplástico, almidón de yuca, almidón acetilado, materiales biodegradables, plastificación de almidón, materiales renovables.
1. INTRODUCTION
Recent research efforts in the area of polymeric materials have focused on the development of biodegradable materials obtained from renewable resources such as starch, proteins, hydroxyalkanoates, etc., which are liable to complete biodegradability when subjected to composting conditions [1, 2, 3]. Nowadays, due to the worldwide decrease of oil production, the search for new materials from renewable resources becomes crucial for substituting traditional synthetic polymers. Among these materials stands out thermoplastic starch (TPS) since the starches are the most abundant and cheapest materials that come from renewable resources [1, 4].
Starch consists of two major components: amylose, a mostly linear α-D(1,4)- glucan and amylopectin, an α-D-(1-4)-glucan which has α-D(1,6) linkages at the branch point. The linear amylose molecules of starch have a molecular weight of 0.2–2 million, whereas the branched amylopectin molecules have molecular weights as high as 100–400 million [5]. Most often the structure of starch is chemically modified in order to decrease the number of hydroxyl groups in the search for a number of possible applications. Thereby, starch has been treated by hydroxipropylation [6], oxidation [7] and acrylic grafting, mainly. However, in recent years, there has been a considerable research effort with acetylated thermoplastic starch, because of the experience acquired at food applications where acetylation is the second most studied treatment after crosslinking [8]. Acetylation is the esterification of starch with acetic anhydride, used to facilitate the formation of acetates groups, which has been performed mainly to adjust the viscosity of food and to improve its stability and its resistance to degradation as compared to untreated starch. Also, the incorporation of acetate groups reduces the interaction between chains and improves the swelling power and the solubility of starch granules. The change of the physicochemical properties of starch is proportional to the substitution degree of the formed acetate groups [9].
In order to change a native starch into a bioplastic material it is necessary to break up its semicrystalline granular structure [10]. A starch without the appropriate additives (plasticizers) does not possess the necessary properties to perform as a thermoplastic. The plasticizers increase the flexibility of starch because of their ability to reduce the formation of hydrogen bonds and increase molecular separation [11]. In the field of food science, water is considered to be the best plasticizer. However, in the case of TPS, water is included as an aid to the main additive, glycerol being the most studied. In order to improve the poor mechanical properties of starch and to reduce its water absorption, several studies found in the literature deal with different aspects concerning the improvement of plasticizers [2, 12], the more stable biodegradable polymer blends [13, 14], the incorporation of natural fibers [15, 16] and as mentioned before, the chemical modifications by acetylation is the most studied. It is worth mentioning that most of these studies were performed on starches obtained from corn, barley, potato, and rice.
The purpose of this paper is to characterize two biodegradable types of thermoplastic starch obtained from natural and acetylated cassava starch (Manihot sculenta crantz) by their physicochemical, thermal and mechanical properties.
2. EXPERIMENTAL METHODOLOGY
2.1 Materials Cassava starch samples, natural food grade and acetylated industrial grade, were kindly provided by Industrias del Maíz S.A. (Corn Products Andina Colombia), from Cali, Colombia and were used as received. The glycerol used was industrial grade.
2.2 Preparation of thermoplastic starch
Similar to the process reported by Huang [3] and Ma [17], 2006, both the cassava starch and the acetylated one were first dried for 24 hours at 80°C and then mixed with glycerol in a 70:30 weight ratio using a Black and Decker high speed mixer, until the lumps disappeared (around five minutes). The mixture was then stored in a sealed container for 72 hours. Finally, it was plasticized using a co-rotating twin screw extruder coupled to a Brabender plasticorder Model PLE 330, equipped with a 32 mm conical cylinder and an L/D ratio of 13. The rotating speed was kept at 45 rpm and the temperature profile used was 115, 125, 130 and 135 ºC for all three zones of the screw and the head respectively.
2.3 Fourier Transform Infrared Spectroscopy (FTIR) Fourier Transform Infrared Spectroscopy analysis was performed on a Nicolet model Protégé 460 magna IR spectrometer. The analysis of the milled starch (natural and acetylated) was carried out with analytical grade KBr pellets using the transmission mode. The spectra were recorded with 4 cm-1 resolution and 100 scans. In the case of the TPS, a microscope with an accessory for attenuated total internal reflectance (ATR) spectroscopy was used.
2.4 Thermogravimetric analysis (TGA)
Thermal stability was evaluated using a Perkin Elmer TGA 7 in a temperature range between 50 and 650 °C, with a heating rate of 10 ºC/min and an inert atmosphere, nitrogen gas flow of 20 ml/min. The average weight of the evaluated samples was 7 mg.
2.5 Scanning electron microscopy (SEM)
The morphology of the milled starch particles and the failure surfaces of both the natural and acetylated thermoplastic starches were observed using a scanning electron microscope JEOL SEM Model LV 5400. The samples were coated with gold prior to the analysis.
2.6 Mechanical properties Tensile mechanical properties of both, the natural and acetylated thermoplastic starches were determined after the room conditioning of the samples. The testing was carried out only when the hygro-thermal equilibrium had been achieved at 54 % RH and a temperature of 25 ºC. The tensile testing was performed using a Shimadzu Model AG-1 100 KN universal testing machine equipped with a 500 N load cell. Type IV samples were prepared according to the ASTM D-638 standard specifications using a cross-head speed of 5 mm/min.
2.7 Moisture absorption Isotherms The thermoplastic starch samples were dried in an oven at 80 °C during 12 hours and then placed in a desiccator with an aqueous solution of hexa hydrated magnesium nitrate in order to maintain a relative humidity of approximately 54 ± 2 %. Weight gain data were obtained as a function of time (Pt) at a temperature of 25 ± 2 ºC, moisture absorption (H) was calculated as a percentage, taking the weight obtained right after oven drying (Ps) as the initial value, according to (1).
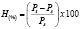 (1)
(1)2.8 Dynamical mechanical analysis (DMA) A Dynamical Mechanical Analyzer (Perkin Elmer DMA 7) was used for the determination of the second order transitions of both the natural thermoplastic starch and the acetylated thermoplastic starch, using the extension mode. The specimen size was 2 mm x 1 mm x 6 mm. The samples were analyzed at a temperature range between -100 and 60 ºC (cooling with liquid nitrogen), using a heating rate of 5 °C/min, a frequency of 1 Hz and a gas nitrogen flow of 20 ml/min.
3. RESULTS AND DISCUSSION
3.1 Fourier transform infrared spectroscopy (FTIR) The FTIR spectra for both the natural and acetylated starches are shown in Figure 1. The assignment of the corresponding bands for both starches is presented in Table 1. The main difference in the spectra of the milled starches is attributed to the appearance of new bands at 1740, 1375 and 1240 cm-1, characteristic of the acetate group formed in the acetylation process. A typical reaction for such a process is illustrated in Figure 2. Here, the structure of the repeat units where the chemical modification is performed is presented. These bands can also be observed in the spectrum corresponding to the acetylated TPS. In Figure 3, the interval from 800 to 1400 cm-1 of the starches studied is shown. Smits et al. [18] pointed out that the band for 1047 cm-1 is related to the crystalline phase of starch, while the band at 1022 1047 cm-1 is related to the amorphous one. From a reference line drawn between 1180 and 880 cm-1, the index of crystallinity was calculated as the band height ratio (H1047/H1022).

Figure 1. FTIR spectra of natural and acetylated starch and natural and acetylated TPS
Table 1. Typical bands in infrared spectroscopy of thermoplastic starch [8, 19]


Figure 2. Scheme of a starch acetylation process

Figure 3. FTIR amorphous and crystalline regions for natural and acetylated starch and natural and acetylated TPS
It was found that the crystallinity indexes were 0.911 and 0.821 for the natural and acetylated milled starches respectively, and 0.694 and 0.716 for the natural and acetylated thermoplastic starch. These results indicate, as expected, that the extrusion processes decrease the crystallinity of both types of starch, more noticeable for natural starch compared with the acetylated starch. The effect of the acetylation on the morphology of starches is more complex. On one side, the acetylation, at least at the surface level, partially decreases the arrangement of the starch granules, in agreement with the distortion related to the loss of symmetry upon the replacement of the hydroxyl groups –OH by the acetate groups –CH3OCO, which are larger.
Also, when comparing the crystallinity indexes, between the thermoplastic starches, the acetylation process appears to give place to a highly ordered TPS, when compared to the natural starch. This behavior tends to suggest that the interactions between starch and glycerol play an important role in the plasticization process.
3.2 Thermogravimetric analysis (TGA) The loss of mass occurring along the temperature range selected for this study, revealed that, with the plasticization process of starch, thermal stability is lost, as shown in Figure 4; such a decrease is mainly attributed to the incorporation of glycerol, a low molecular weight molecule, whose evaporation and/or decomposition occurs at lower temperatures than the starches. Figure 5 shows the derivative of the thermogravimetric analysis curves.

Figure 4. TG thermogram for natural and acetylated starch and natural and acetylated TPS

Figure 5. DTG thermograms for natural and acetylated starches and natural and acetylated TPS
The temperature for the higher rate of mass loss of these materials can clearly be observed. It can also be seen that the plasticized starch presents small shifts to the right for the peak and that the acetylation process induces a shift of the peak to the left for both the plasticized starch and the milled-like granular structure. It is observed that for glycerol there is a peak value at 209 °C, where the largest mass loss occurs, as depicted by an almost vertical drop of the thermogram in Figure 4.
The kinetic parameters for the materials can be calculated from the model proposed by Broido [20], as shown in (2).
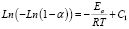 (2)
(2)Where:
a = Reaction extent of the component of the sample being degradedSimilarly, the reaction extent a is determined from the initial (w0), and final (w¥), and at any given time (wt) weights of the sample, using (3).
Ea = Activation energy
R = Universal Gas Constant
T = Temperature
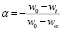 (3)
(3)The activation energy can be estimated from the calculation of the slope of the curve given by (2); it should be pointed out that, in the graph for Broido’s model there are three different lineal regions in the temperature range which are also associated with three different activation energies.
These regions have also been reported by other researchers [18, 21] in studies of dry and plasticized starch. Figure 6 shows the values of the activation energy for the central temperature region (290 – 340 ºC), which are shown in Table 2 where the peak value of the curve for the derivative occurs.

Figure 6. Estimation the activation energy using Broido’s method
Table 2. Mechanical and thermal properties of the natural and the acetylated starches

The value obtained for the activation energy for natural cassava starch was 129 kJ/mol. A value of 102 kJ/mol was reported by Alvarez and Vazquez [22] for corn starch calculated using the Kissinger method. It should be pointed out that the calculated values for the kinetic parameters vary according to the type
of atmosphere, the shape and amount of the sample; the flow and heat rate; and the method used for the calculations. In the case of the thermoplastic starch, the secondary interactions between the polymeric chains are smaller, compared with the non-plasticized starch, due to the presence of glycerol. Therefore, it is expected that the activation energy for the thermal degradation process of the TPS should be smaller than the one for the milled starch.
The value of Ea for natural TPS, was 51.3 kJ/mol, a value of 63.3 kJ/mol was reported by Valles et al. [21], for a mixture of thermoplastic starch, also plasticized with glycerol. This value was also calculated using Broido’s model for a temperature interval similar to the one used in this study.
3.3 Scanning electron microscopy Figures 7 (a) and 7 (c) show micro photographs of the morphology for natural and acetylated cassava starch particles, used in the plasticization process, respectively. As stated by Wurzburg [23], the geometry of this type of starches is sphere-like, as opposed to those from other botanical sources which posses a polygonal shape. The presence of the plasticizer and the resulting shear stress led to the breakage of the granular structure of starch. However, as seen in the microphotographs Figures 7(b) and 7(d), there still are a few granules that are not totally fragmented; this is an indication that the processing conditions can still be improved. The last microphotographs were taken from the failure cross-section of tensile test samples.

Figure 7. SEM micrographs of natural milled (a) and acetylated starch (c); tensile fracture surface of natural (b) and acetylated thermoplastic starch (d)
3.4 Mechanical properties The tensile strength found for the natural and the thermoplastic starch are low compared to the 5.5 MPa value reported by Huang et al. [2] for corn starch with similar glycerol contents. It should be pointed out that they used a higher strain rate (10 mm/min) and a lower relative humidity (50 %) than those used in this study (5 mm/min y 54 %). Also, as reported by Mathew [24], for a system of corn starch with similar glycerol contents, the tensile strength, elastic modulus and strain at break were 0.23 MPa, 38 MPa, and 14 %, respectively. Ruiz [25] reported a tensile strength of 0.38 MPa, and strain at breaking point of 69 % for a thermoplastic starch with a 65 % modified cassava starch and 35 % glycerol. It is important to point out that the mechanical properties of the TPS vary with time, and with the temperature and relative humidity they are exposed to [26]. This is attributed to the water plasticization and retrogradation phenomena. These are is the main reasons why there are always differences in the reported values for this type of characterization.
The typical stress-strain curves for both natural and acetylated TPS are shown in Figure 8, and the average values of five specimens tested can be seen in Table 2. Figure 8 shows that the mechanical behavior of both TPSs is different. The acetylated TPS has lower tensile break strength and a higher tensile break strain compared to natural TPS. This finding suggests that the role of glycerol during the plasticization was more effective for the chemically modified starch than for the natural one. In the case of the tensile modulus, no statistical differences were found.

Figure 8. Tensile strength vs strain at breaking point curves for natural and acetylated thermoplastic starch
3.5 Isotherms of moisture absorption Figure 9 shows the curves for the isotherms of moisture absorption from the thermoplastic starches. It was found that the moisture absorption for the conditions in this study was of the order of 10 %, and a slight decrease in the absorption was evident for the acetylated material.

Figure 9. Moisture absorption isotherm at 25 ºC and 54 % relativity humidity for natural and acetylated thermoplastic starch
As expected, this decrease of the absorption suggests that there is a decrease in the number of hydroxyl groups, attributed to the acetylation of the starch, thus influencing the capacity for the formation of bonds between the material and water molecules from the environment.
Furthermore, a noticeable decrease in the absorption of moisture could be expected for the starches presenting higher percentages of substitutions. Similar values of moisture absorption have been reported by Curvelo [15] for a starch plasticized with 30 % glycerol; as reported in these studies, an absorption of approximately 9 % is generated for equilibrium conditions when the relative humidity and the temperature are kept at 43 % and 25 ºC respectively.
3.6 Dynamical mechanical analysis Figure 10 shows the curves for Tan d obtained from a DMA for both, the natural and acetylated TPS. As can be seen, there are two second order transitions β and α, which have been amply reported in the literature [27].

Figure 10. DMA Tan d curves for natural and acetylated thermoplastic starch
The first peak is associated with local movements attributed to the glycerol molecules, while the second transition is commonly denoted as the glass transition and is related to the movements when the energy is high enough to generate conformational changes at the molecular chain level. In a similar study Da Roz [28] evaluated different plasticizers (ethilenglycol, sorbytol, polyethilenglycol, 1-4 butanodiol, etc.) used as additives for corn starch TPS; he reported transitions in similar intervals to those obtained via DMA and shown in Figure 10. On the other hand, we observe that the chemical modification of starch at low substitution degrees generates a decrease in the associated temperatures related to the transitions, suggesting that, in agreement with the analysis of the moisture absorption isotherms, little effect exist in the decrease of the secondary interactions between the starch molecules, also attributed to the decrease in the number of hydroxyl groups.
4. CONCLUSIONS
The acetylation treatment of cassava starch, contributed to the increase of the amorphous region of the material. This was made evident by the relative decrease of the IR band at 1047 cm-1 associated with the crystalline regions of the material. The water absorption capability of the acetylated starch was smaller than that of the natural one despite the lower acetylation degree. This fact suggests that an increase in the degree of chemical modification could contribute to further diminishing the capability of moisture absorption of the TPS. Although the acetylation process decreases the mechanical strength of the TPS it seems that the better interaction of the glycerol with the modified starch would allow us to decrease it in order to improve the TPS mechanical properties.
5. ACKNOWLEDGEMENTS
This work was performed with the support of the Centro de Investigación Científica de Yucatán A.C. (CICY) México, the Universidad del Valle, COLCIENCIAS and the Centro de Excelencia de Nuevos Materiales (CENM), Colombia. The authors also express their gratitude to Industrias del Maíz for having provided the cassava starch and Q.I. Tanit Toledano-Thompson for the SEM micrographs.
REFERENCES
[1] GUAN, J., ESKRIDGE, K. AND HANNA, M. Acetylated starch-polylactic acid loose-fill packaging materials, Industrial Crops and Products, 22, 109-123, 2004. [ Links ]
[2] HUANG, M., YU, J. AND MA, X. Ethanolamine as a novel plasticizer for thermoplastic starch, Polymer Degradation and Stability, 90, 501-507, 2005. [ Links ]
[3] VILLADA, H. Influencia de mezclas de almidón agrio, perfil de temperatura y velocidad de tornillo de un extrusor sencillo en la producción de almidón termoplástico, su caracterización fisicoquímica, mecánica, microestructural y comportamiento durante el almacenamiento [Tesis Ph.D.]. Universidad del Valle, Facultad de Ingeniería, 2005. [ Links ]
[4] LAI, S., DON, T. AND HUANG, Y. Preparation and Properties of Biodegradable Thermoplastic Starch/Poly(hydroxyl butyrate) Blends, Journal of Applied Polymer Science, 100, 2371-2379, 2006. [ Links ]
[5] HUANG, M., YU, J. AND MA, X. Ethanolamine as a novel plasticizer for thermoplastic starch, Polymer Degradation and Stability, 90, 501-507, 2005. [ Links ]
[6] KIM, D-H., NA, S-K. AND PARK, J-S. Preparation and Characterization of Modified Starch-Based Plastic-Film Reinforced with Short Pulp Fiber. I. Structural Properties, Journal of Applied Polymer Science, 88, 2100-2107, 2003. [ Links ]
[7] ACIOLI, R. The effect of physical aging, Starch particle size, and Starch oxidation on thermal-mechanical properties of Poly(lactic acid)/Starch composites, [Thesis PhD]. College of Agriculture. Kansas State University, 2006.
[8] WANG, X. et al. Study on the morphology, crystalline structure and thermal properties of yam starch acetates with different degrees of substitution, Science in China Series B: Chemistry, 51, 9, 859-865, 2008. [ Links ]
[9] NAVDEEP, S. AND NARPINDER, S. Characteristics of acetylated starches prepared using starches separated from different rice cultivars, Journal of Food Engineering, 70, 117-127, 2005. [ Links ]
[10] THIRE, R., SIMAO, R. AND ANDRADE, C. Investigation of the Surface Morphology of Plasticized Corn starch Films, Act Microscopic, 12, 1, 175-179. 2003. [ Links ]
[11] MALI, L., SAKANAKA, F. AND YAMASHITA, M. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect, Carbohydrate Polymers, 60, 283-289, 2005. [ Links ]
[12] MA, X., YU, G. AND WAN, J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch, Carbohydrate Polymers, 64, 267-273. 2006. [ Links ]
[13] AVEROUS, L., MORO, L., DOLE, P. AND FRAINGANT, C. Properties of thermoplastic blends: starch- polycaprolactone, Polmer, 41, 41574167, 2000. [ Links ]
[14] SARAZIN, P., Ll, G., ORTS, W. AND FAVIS, B. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch, polymer, 49, 599609, 2008. [ Links ]
[15] CURVELO, A., CARVALHO, A. AND AGNELLI, J. Thermoplastic starch-cellulosic fibers composites: preliminary results, Carbohydrate Polymers, 45, 183-188, 2001. [ Links ]
[16] MA, X., YU, J. AND KENNEDY, J. Studies on the properties of natural fibers-reinforced thermoplastic starch composites, Carbohydrate Polymers, 62, 19-24, 2005. [ Links ]
[17] MA, X., YU, G. AND WAN, J. Urea and ethanolamine as a mixed plasticizer for thermoplastic starch, Carbohydrate Polymers, 64, 267-273. 2006. [ Links ]
[18] SMITS, A., RUHNAU, F., VLIEGENTHART, J. AND VAN SOEST, J. Ageing of Starch Based System as Observed with FTIR and Solid State NMR Spectroscopy, Starch/Starke, 50, 478483, 1998. [ Links ]
[19] MANO, J.F., KONIAROVA, D. AND REIS, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability, Journal of Materials Science: Materials in Medicine, 14, 127-135, 2003. [ Links ]
[20] BROIDO, A. A Simple, Sensitive Graphical Method of Treating Thermogravimetric Analysis Data, Journal of Polymer Science, A-2, 7, 1761. 1969. [ Links ]
[21] VALLÉS, A. et al. Thermal Analysis Characterization of the Degradation of Biodegradable Starch Blends in Soil, Journal of Applied Polymer Science, 96, 358-371, 2005. [ Links ]
[22] ALVAREZ, V. AND VAZQUEZ, A. Thermal degradation of cellulose derivates/starch blends and sisal fibre biocomposites, Polymer Degradation and Stability, 84, 13-21, 2004. [ Links ]
[23] WURZBURG, O. Modified Starches: Properties and Uses, CRC Press, 2000. [ Links ]
[24] MATHEW, A. AND DUFRESNE, A. Plasticized Waxy Maize Starch: Effect of Polyols and Relative Humidity on Material Properties, Biomacromolecules, 3, 1101-1108. 2002. [ Links ]
[25] RUIZ, G. Obtención y caracterización de un polímero biodegradable a partir del almidón de yuca, Ingeniería y Ciencia, 2, No. 4, 5-28. 2006. [ Links ]
[26] MINA, J., VALADEZ, A., HERRERA-FRANCO, P. AND TOLEDANO, T. Influencia del tiempo de almacenamiento en las propiedades estructurales de un almidón termoplástico de yuca (TPS), Ingeniería y Competitividad, 11, No. 2, 95-106. 2009. [ Links ]
[27] LOURDIN, D., BIZOT, H. AND COLONNA, P. Antiplasticization in Starch-Glycerol Films?, Journal of Applied Polymer Science, 63, 8, 1047-1053, 1997. [ Links ]
[28] DA, R. The effect of plasticizers on thermoplastic starch compositions obtained by melt processing, Carbohydrate Polymers, 63, 417-424, 2006. [ Links ]
For further details log on website :
http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0012-73532011000200020





No comments:
Post a Comment