Journal of Encapsulation and Adsorption Sciences
Vol.06 No.04(2016), Article ID:72533,15 pages
10.4236/jeas.2016.64010
Vol.06 No.04(2016), Article ID:72533,15 pages
10.4236/jeas.2016.64010
Author
Selma Chraibi1*, Hamou Moussout2, Fatima Boukhlifi2, Hammou Ahlafi2, Mohammed Alami1
1Equip Materials, Metal industry and Engineering of the Processes, National School of Arts and Trades, Meknes, Morocco
2Equips Materials and Applied Catalysis, Faculty de Science, Meknes, Morocco
Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
Received: October 2, 2016; Accepted: December 2, 2016; Published: December 5, 2016
Calcined eggshells (CES) were tested as adsorbent at a low cost for the removal of phenol from the waste water. The shells of Eggs extracts from waste were washed and then dried at a temperature of 60˚C and finally calcined in an oven at the atmospheric air in several temperatures 200˚C, 400˚C, 600˚C, 800˚C, 1000˚C. The chemical composition of the obtained adsorbs was analyzed by the X-ray diffraction. The isothermal study of adsorption of the phenol was realized for the various adsorbates. It showed that the biggest efficiency of the elimination was attributed to the calcined eggshells to 1000˚C with a percentage that reached 37%. The kinetics of adsorption were described by the first rate model. The intra particular distribution is a significant step in the adsorption process of phenol on calcined eggshells (CES). The separation factor gives a favorable adsorption of phenol on the CES.
Keywords:
Calcinations, Adsorption, Eggshells, Phenol, Isothermal Study
Phenol compounds are present bactericidal molecules in industrial effluents which can come from many activities. They are the priority concerns of pollutants because of their toxicity and their potential accumulation in the environment. Phenols are introduced into the wastewater effluent from several industries such as tar industry, gasoline, plastics, rubber, disinfectants, pharmaceutical products and agro-food activities [1] . Various methods have been proposed for the treatment of wastewater containing organic and inorganic pollutants. These processes are based on the principles of the precipitation, coagulation, oxidation, sedimentation, filtration, adsorption, osmosis and the ion exchange, etc. The method of separation most frequently used in water treatment is adsorption including adsorption on activated carbon. The adsorption mechanism of these phenol compounds is still not known and it is even more difficult to treat mixtures of these molecules [2] .
The major disadvantage of the activated carbon is associated with the cost; because of that several researches directed their interests in the production of adsorbents, which have significant capacity to absorb and remove phenols at low cost. The clay is one of the alternative solutions in the activated charcoal; its adsorption capacity result is due to its high surface area and its exchange capacity. Thus, studies [3] and [4] used bentonite, kaolinite and basalt clay modified to adsorb phenol.
The abundance and the availability of agricultural by-products make them good sources of raw materials for natural adsorbents. Rice Bark, an agricultural waste, has been reported as a good adsorbent for many organic and inorganic components including phenol [5] . The rice husk treated chemically was tested to remove phenol in the synthetic wastewater. There are others, such as beet pulp carbonized at 600˚C [6] , activated charcoal pistachio shell which proved an excellent adsorbent to 69.6% removal of phenol and 4-chlorophenol [7] , also marine products such as chitin, which was prepared from the shells of crabs [8] . Eggshell, abundant in our waste MOROOCO, offers us a natural adsorbent with a very low cost. Several studies have examined the potential for adsorption of pollutants. Rarely used, the egg shell was used to adsorb a different dye such as yellow reactive dye 205 [9] , the Crystal Violet [10] , cationic and anionic dyes, blue 51 orange basics acids [11] of the textile dyes Direct (DR80) and acid (AB25) [12] . From these studies, it was found that there is a relatively high adsorption capacity over other adsorbents.
Several studies have worn on the adsorption of Cu2+ and Fe2+ from aqueous solutions on the egg shell powder [13] [14] . Other studies have optimized adsorption of phosphates on the ESC. The calcinations showed that it allows increasing the adsorption capacity [15] . Recent studies have achieved the adsorption of phenol on eggshells uncalcined [16] . The purpose of this work is the study of the removal of phenol in aqueous solution by calcining eggshells (CES) waste extracts [17] . It also relates to the optimization of the retention of phenol on the eggshells calcined at different temperatures of 200˚C to 1000˚C. The effect of various conditions and parameters were studied in batch experiments [18] .
2. Materials and Methods
Calcined Eggshell (CES): eggshell was collected from the waste of restaurants and their storage. It was cleaned with demineralized water several times and finally dried in an oven (60˚C) for 24 hours [12] . Eggshells were prepared and sieved with a sieve of 425 μm. Masses of the adsorbent were calcined in an electric furnace treatment ELTI CFZ 1. This furnace seven Fofumi can rise to a maximum temperature to 1100˚C, which allowed us to calcine our shells at different temperatures at 200˚C, 400˚C, 600˚C, 800˚C and 1000˚C [15] for 2 h.
The adsorption kinetics were studied according to previous work [16] . It was measured by the amount of adsorbed phenol determined at certain time intervals (5, 15, 30, 60, 90, 120, 150, 180, 210, 240, 270 and 300 min). At these intervals, samples were removed and filtered. The concentration of phenol in the liquid phase was measured with the UV spectrophotometer-Visible CECIL 2021 at λmax = 270 nm. It gave the sample absorbance.
Other adsorption experiments were carried out at different concentrations of phenol solutions for drawing the adsorption isotherm. It used an optimum CES quantity (100 mg). The concentration of the phenol solution was between 0.001 and 0.008 mol/l with a stirring speed of 1000 revolutions per minute, as the temperature was room temperature ± 25˚C with a pH (≈8) solution for 150 min to reach equilibrium conditions. The results were verified with the adsorption Langmuir and Freundlich models.
Calcination eggshell is an interesting setting to explore for adsorption of phenol. Eggshells were calcined at temperatures ranging from 200˚C to 1000˚C for 2 h in an oven at the atmospheric air.
3.1. Analysis of the Mass Loss of CES
The results of monitoring of the mass loss as a function of the calcination temperature were grouped in Figure 1. This figure shows two distinct stages of weight loss: a step at temperature below 600˚C and another between 600˚C and 850˚C. Before calcination,
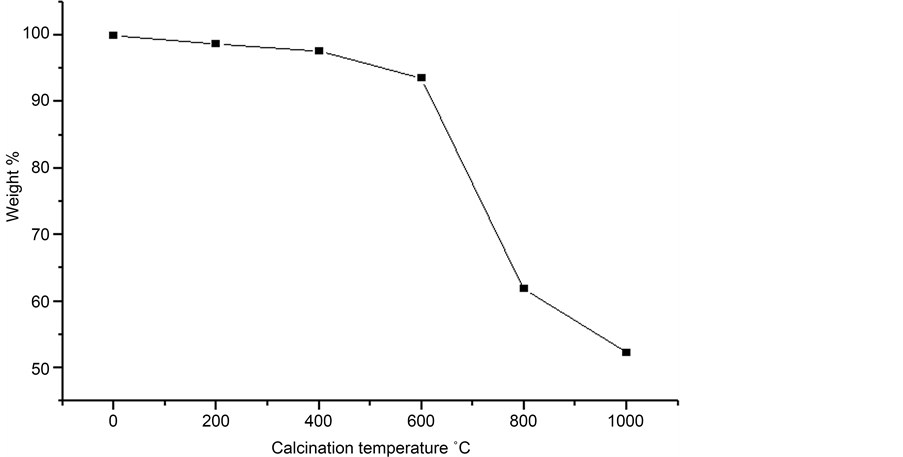
Figure 1. Mass loss of ESCs depending on the calcination temperature.
ESCs consist primarily of CaCO3 (91.94%) in addition to other elements; i.e. Si, S, Mg, P, Al, K and Sr [19] with a very low percentage. The first phase may be explained by the evaporation of adsorbed water and other molecules. The second step involves the loss of the largest weight corresponding to 40% of weight of 600˚C to 850˚C. This variation is due to the phase change from CaCO3 to CaO phase which could be confirmed by X-ray diffraction. The calcination was continued to ensure complete conversion to CaO. The decomposition of the carbonate was accompanied by evolution of CO2, according to the following endothermic reaction [20] :
3.2. X-Ray Analysis
The calcined samples were characterized by X-ray diffraction (Figure 2). The relative curvature as calcinations before eggshells showed a maximum peak at 2θ = 29.48˚, indicating that the calcite (CaCO3) is a main phase of the shell. The peaks relating to the CaO phase were detected for the shells calcined at 800˚C and 1000˚C. The 2θ values of CaO peaks are: 33.90˚; 39.24˚; 43.23˚; 47.26˚; 48.27˚; 50.79˚.
We note that the absence of the maximum peak on the calcite phase (2θ = 29.48) in the shells calcined at 800˚C, which implies that the CaCO3 layer was completely decomposed into CaO. These results are consistent with previous studies [19] [20] . Information relating to the peaks is given in Table 1. In the curves for 200˚C, 400˚C, 600˚C, there is the peak 2θ = 29.48˚ with a lower intensity and the appearance of other peaks as 2θ = 39.44˚; 47.61˚; 48.79˚. We conclude that the phases obtained after calcinations at 200˚C, 400˚C and 600˚C are a mixture of the two phases CaCO3 and CaO.
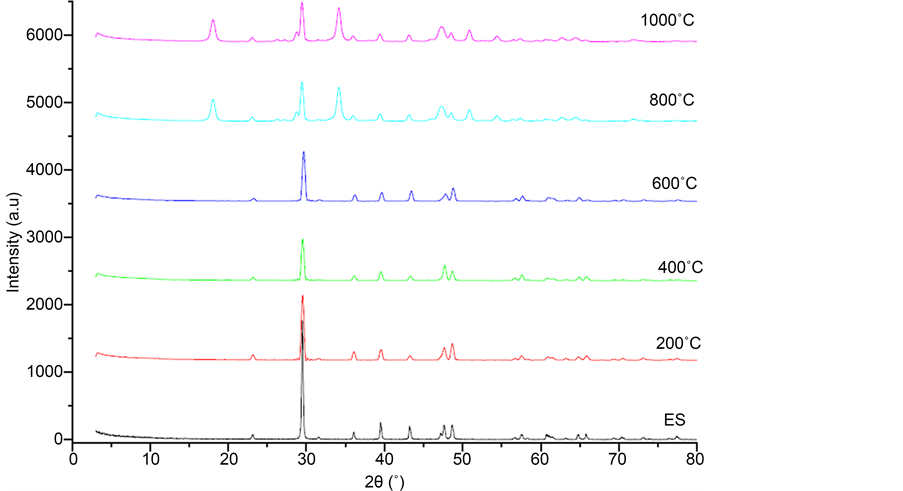
Figure 2. X-ray diffractograms eggshells before and after calcination at different temperatures.
Table 1. List of some peaks obtained by X-ray diffraction for eggshells.
3.3. Adsorption Kinetics
The phenol removal efficiency was assessed by determining the adsorption capacity and the percentage removal of phenol. For this, the adsorption capacity of phenol was calculated from the relation [21] [22] :
where Q (mg/g) is the equilibrium capacity adsorption, C0 and Ce (mg/L) are, respectively, the initial liquid phase concentration of phenol and the concentration of phenol at equilibrium, V (l) is the volume of the solution, m (g) is the mass calcined shells.
To calculate the percentage of removal of phenol in solutions by CES, we used the following equation:
With C0 and Ce (mg/L) are, respectively, the initial liquid phase concentration and the concentration of the phenol at equilibrium.
According to the results presented in Figure 3, it is clear that by increasing the temperature of calcination of the eggshells, the percentage removal of adsorbed phenol increases as well as the rate of adsorption.
Kinetic studies realized on non-calcined shells [16] found a slow equilibrium of time equal to 210 min. However, for CES, the time equilibrium decreased in 90 min for CES at 800˚C and 1000˚C (CaO) and in 150 min for CES at 200˚C, 400˚C, 600˚C (CaCO3 + CaO). According to Figure 4, the removal of phenol by CaCO3 + CaO was about 70 mg/g. This value is increased of 10 mg/g for CaO. This can be justified by the pores multiplication [19] and the increase of the pores sizes distribution [15] .
Figure 4 shows the effect of calcination temperature on the adsorption of the phenol. We can observe that the maximum percentage of removal of the phenol is about 37% for calcined shells to 1000˚C. The removal percentage did not vary significantly between all our calcined samples.
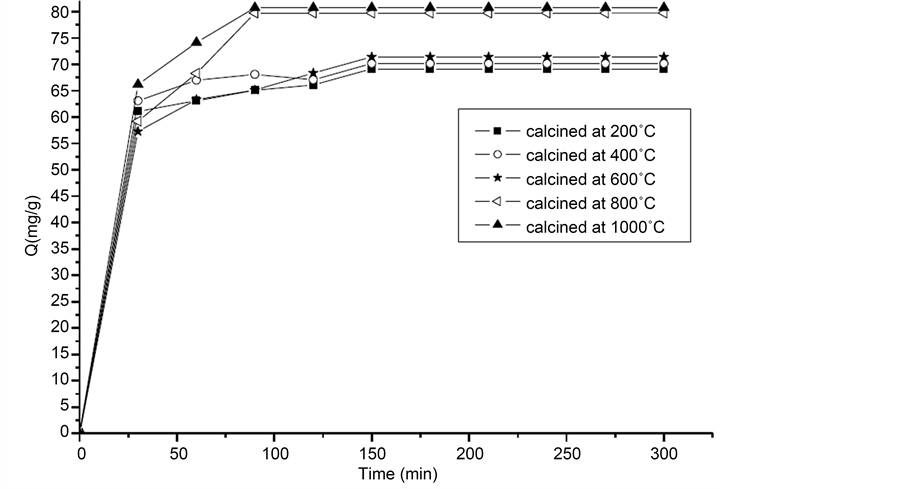
Figure 3. The kinetics of phenol adsorption on calcined shells.
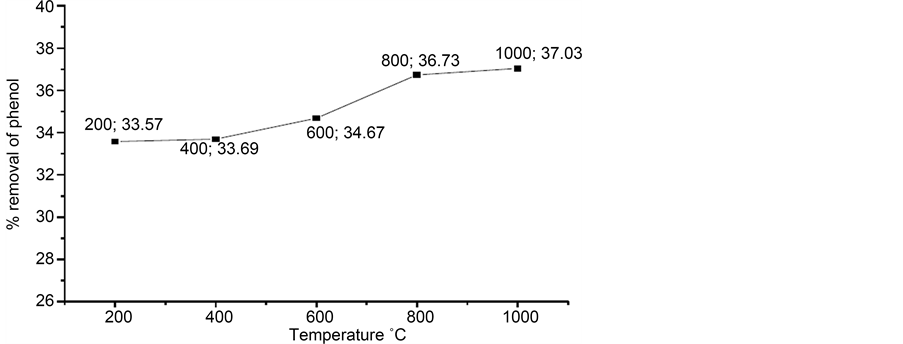
Figure 4. Effect of calcination temperature on the adsorption of phenol.
3.3.1. Modeling of First and Second Order
For the examination of the mechanisms that control adsorption, such as the chemical reaction and the diffusion control, several kinetic models are used to test experimental data. The orders concerning the adsorption on bioadsorbants most quoted in the literature are:
The model pseudo-first order Lagrange which is based on the hypothesis that the rate of variation of solution adsorbed according to time is proportional to the difference between adsorption capacity balance and the amount adsorbed at time t.
The pseudo-first order after the integration is expressed by [16] :
where k1 is the constant of speed of pseudo-first-order (min−1) and qe (mg∙g−1) is the adsorption capacity for the balance and qt (mg∙g−1) is the amount of phenol adsorbed at each time t.
After the integration, the pseudo second order model is expressed as follows:
where k2 (mg∙g−1∙min−1) is the constant of speed of pseudo-second order. The values of qe, k1and k2 for the different adsorbents were calculated from the graphs in Figure 5 and Figure 6 and the results are presented in Table 2.
By comparing the results after linear modeling, the adsorption kinetics for CES with

Figure 5. Drawing the linear form of the first-order kinetic model for calcined shells.
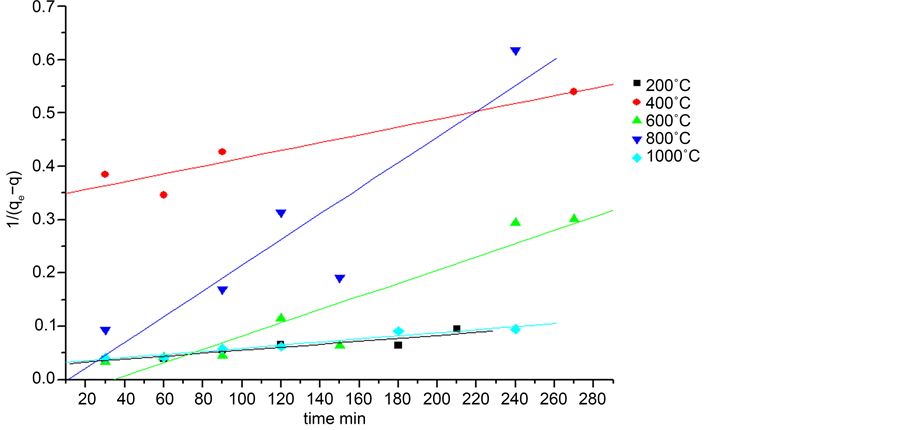
Figure 6. Drawing the linear form of the second-order kinetic model for calcined shells.
Table 2. Parameters of the adsorption kinetics of phenol for both models.
both models, it is found that the adsorbed amount increases with the increase of the calcination temperature, the highest correlation coefficient is registered for the first-order model. As a result, this model describes or gets closer to our study.
3.3.2. Study of Diffusion Processes
The diffusion process usually consists of four stages, which may be either independent of each other, or simultaneously. The first step is the movement of the solution to the solid surface. The second is the diffusion through the interparticle spaces (external diffusion). The third relates to the intraparticle diffusion, and finally is the chemical reaction surface between the surface features of the adsorbent and the active groups of the phenol [23] .
1) External diffusion process
To model the external diffusion process, we will use the expression:
Ce: the equilibrium concentration of solute solution; a: area of the solid/liquid interface; V: volume of solution.
The integrated form of the external distribution process:
The plot of  versus time t, give the value of
versus time t, give the value of  and thus to assess
and thus to assess
whether the diffusion step external step is decisive for the entire reaction.
2) Intra-particular diffusion process
The intra-particular diffusion kinetics phrase is often simply presented by equation [23] :
kid constant of intra-particular diffusion rate (m∙L−1∙min−0.5); C: constant that gives an idea about the thickness of the boundary layer. Its values were determined for each adsorbent by the graph of qt versus t1/2 is given in Table 3.
Table 3. Parameters of the diffusion process for the adsorption of phenol by calcined eggshells.
Both graphs in Figure 7 and Figure 8 are linear with a high correlation. It is deduced that the diffusion process at least plays a more important role in the adsorption of the phenol. We also note that in the first minutes of contact, the chemical reaction on the surface begins. The experimental point’s intra particular process aligns with an R2 regression coefficient higher than that of the external diffusion processes. The parameters of both processes for each adsorbent (eggshells calcined at different temperatures) are summarized in Table 3. This indicates that the most influential step in the adsorption of the phenol on calcined eggshell remains the process intra-particular diffusion.
According to the intra-particular diffusion model and the phenomenon of adsorption [24] ; if the curve goes from the source, then we can say that the adsorption is totally controlled by this step. If the curve does not pass through the origin, then the intra-particular diffusion is not the only step that controls the adsorption, but with a certain degree of control of the boundary layer.
Thus, we can deduct that the intra-particular diffusion is a limiting step which controls the speed of the phenol adsorption at each time t, but other kinetic models can intervene.
3.4. Adsorption Isotherm
Figure 9 shows the adsorption isotherm of calcined eggshells at different temperatures.
The isotherm type observed is type S. There are in the literature four types of insulated by their appearance [25] [26] . These types can provide explanations of the adsorption mechanisms. S isotherm type is encountered when the adsorption becomes easier to as far as that the solution concentration increases. It also considers that this type reflects a strong competition between water molecules and the molecule studied for the adsorption sites [27] .
Equations (8) and (9) of Langmuir and Freundlich are often used to describe the adsorbent-adsorbate relations. These models allow us to properly describe the parameters and adsorption characteristics and secondly, to understand the nature of adsorption phenomena. [27] . In order to facilitate estimation of the phenol adsorption capacity of the calcined eggshells at different temperatures in the liquid phase, the two adsorption isotherm models [16] , Langmuir and Freundlich, were used according to the following formulas:

Figure 7. Plot of the linear form of external diffusion for the adsorption of phenol by calcined eggshells.
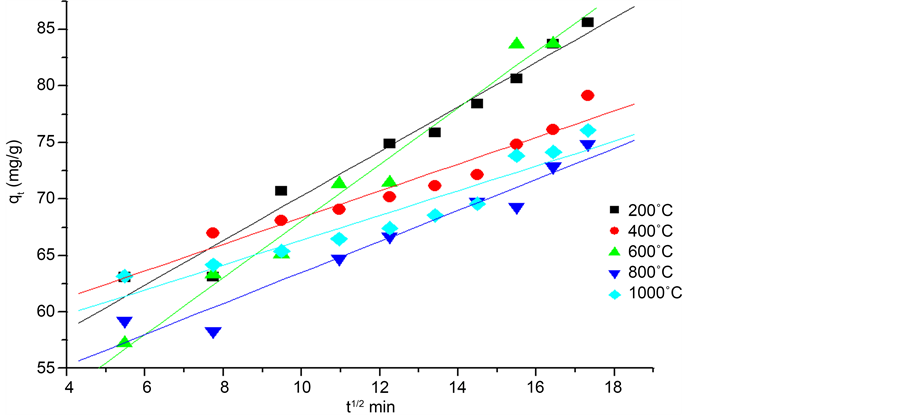
Figure 8. Trace of the linear form of the intraparticle diffusion for the adsorption of phenol by calcined shells.
Langmuir equation with its linear form:
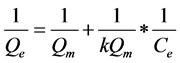 (8)
(8)
With Qm (mg/g) the maximum adsorption capacity; K balance Constant of Langmuir; Qe (mg/g) the amount of phenol adsorbed on CES and Ce (mg/L) concentration of the phenol solution at equilibrium.
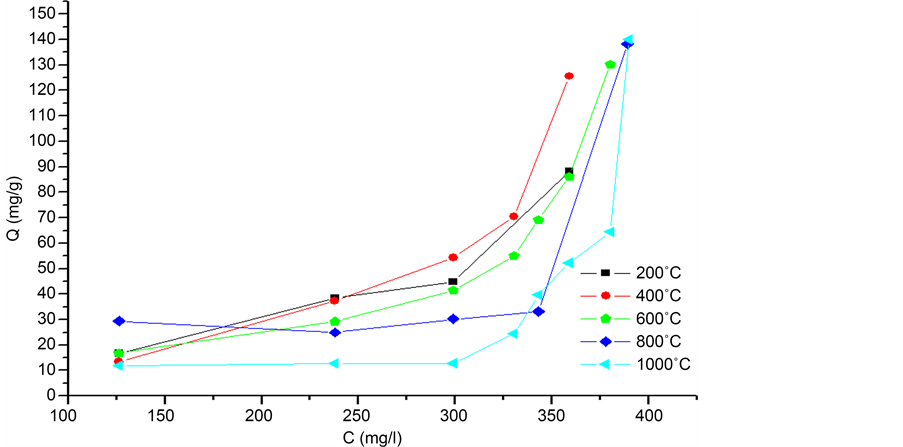
Figure 9. Adsorption isotherm of phenol on calcined eggshells.
Freundlich equation with its linear form:
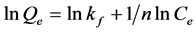 (9)
(9)
With Qe (mg/g) the amount of phenol adsorbed on CES at equilibrium; Ce (mg/L) concentration of the phenol solution at equilibrium; Freundlich constant kf and n the adsorption intensity. This semi-empirical equation gives a better fit for the adsorption from liquids. For this model, the adsorption is carried out on a heterogeneous surface with a non-uniform distribution of the heat of adsorption on the surface.
For optimizing the adsorption system, it is important to establish the most suitable correlation for the adsorption isotherms at equilibrium. By adjusting the experimental points on the two models and be based on the greatest values of coefficient R2. According to the Figure 10 and Figure 11, it appears that the Langmuir best describes the type of our adsorption. For the model of Langmuir, adsorption is carried out on a homogeneous energy surface by the formation of monolayer without any interaction between the adsorbed species [28] .
The data of the two parameters are summarized in Table 4, according to the Langmuir model the adsorption capacity reached 58.83 mg/g for CES at 1000˚C. The adsorption capacity increased with the increase of calcining temperature has been shown by the kinetic study.
The essential feature of the Langmuir isotherm can be expressed by a dimensionless constant named separation factor RL is defined by:
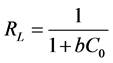 (10)
(10)
where C0 the initial concentration and b the concentration of Langmuir. This coefficient is used to assess the feasibility of adsorption: it assumes that the isotherm is irreversible when RL = 0, favorable when 0 < RL <1, linear when RL = 1 or unfavorable when
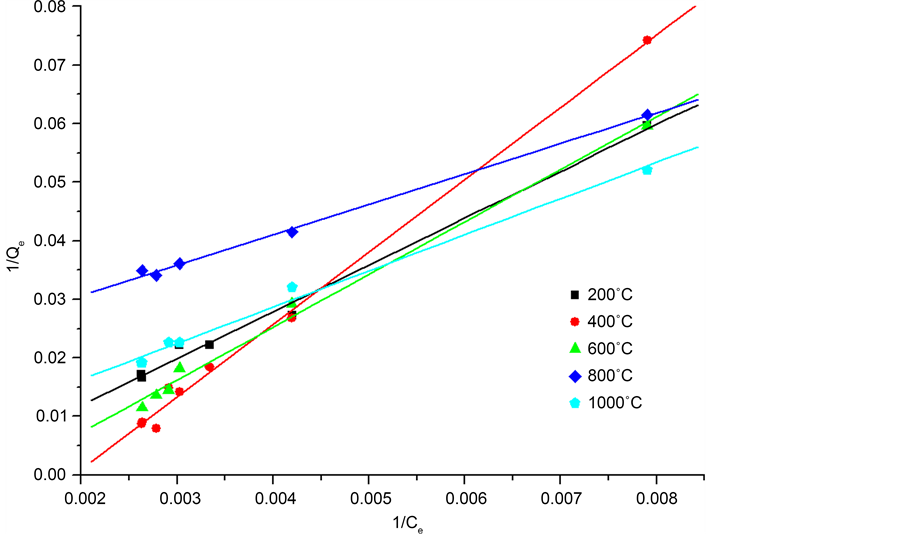
Figure 10. Langmuir linear model of the adsorption of phenol on the calcined eggshells.
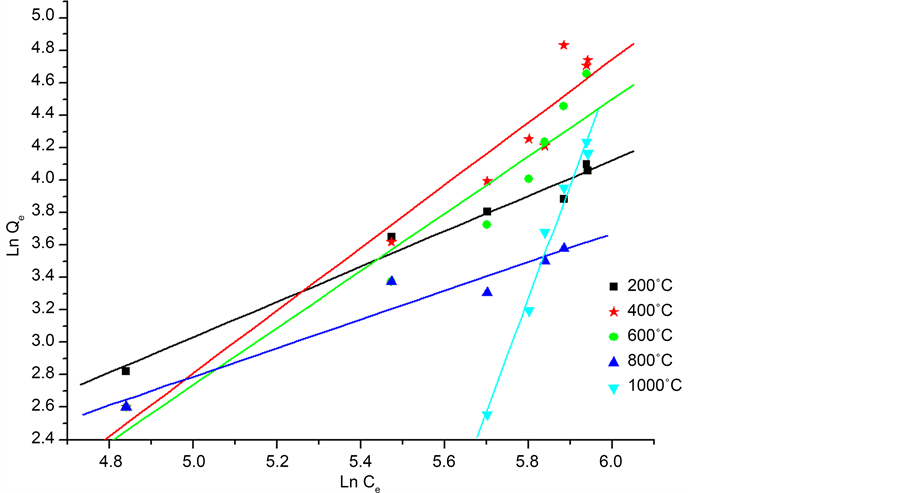
Figure 11. Freundlich linear model of the adsorption of phenol on the calcined eggshells.
RL >1 [28] .
From Table 5, the RL value was calculated for different values of initial concentrations (0.002; 0.005; 0.007 mol/L). For our adsorbents eggshells calcined at 200˚C, 400˚C, 600˚C, 800˚C, 100˚C, the RL value is in the range of 0 - 1, clearly indicating a favorable adsorption in all cases.
Table 4. Characterization parameters of the two model adsorption isotherm of phenol on CES.
Table 5. Values of RL separation factor for different concentration.
This work focuses on the study of the adsorption capacity of the phenol on calcined eggshells (CES) at different temperatures. A highly toxic and organic pollutant in the environment combined. Characterization of our adsorbate showed that the calcination resulted in a change phase; the calcination of rich eggshells CaCO3 at 800˚C gave a calcium oxide CaO. The study of the kinetics and the adsorption isotherm was performed in order to understand and explain the phenol adsorption mechanism on the calcined ES. After calcination of ES, the equilibrium time was reduced by approximately 120 min compared to uncalcined shell. This study follows the Langmuir model, and then one can conclude that the adsorption is carried out on a homogeneous energy surface by the formation of monolayer without any interaction between the adsorbed species. This work shows that the calcination of eggshells is a very interesting optimization setting for the treatment of wastewater polluted with the phenol.
Chraibi, S., Moussout, H., Boukhlifi, F., Ahlafi, H. and Alami, M. (2016) Utilization of Calcined Eggshell Waste as an Adsorbent for the Removal of Phenol from Aqueous Solution. Journal of Encapsulation and Adsorption Sciences, 6, 132-146. http://dx.doi.org/10.4236/jeas.2016.64010
- 1. Calace, N., Nardi, E., Petronio, B. and Pietroletti, M. (2002) Adsorption of Phenols by Papermill Sludges. Environmental Pollution, 118, 315-319.
https://doi.org/10.1016/S0269-7491(01)00303-7 [Citation Time(s):1] - 2. Caqueret, V., Bostyn, S., Cagnon, B. and Fauduet, H. (2008) Purification of Sugar Beet Vinasse-Adsorption of Polyphenolic and Dark Colored Compounds on Different Commercial Activated Carbons. Bioresource Technology, 99, 5814-5821.
https://doi.org/10.1016/j.biortech.2007.10.009 [Citation Time(s):1] - 3. Richards, S. and Bouazza, A. (2007) Phenol Adsorption in Organo-Modified Basaltic Clay and Bentonite. Applied Clay Science, 37, 133-142.
https://doi.org/10.1016/j.clay.2006.11.006 [Citation Time(s):1] - 4. Alkaram, U.F., Mukhlis, A.A. and Al-Dujaili, A.H. (2009) The Removal of Phenol from Aqueous Solutions by Adsorption Using Surfactant-Modified Bentonite and Kaolinite. Journal of Hazardous Materials, 169, 324-332.
https://doi.org/10.1016/j.jhazmat.2009.03.153 [Citation Time(s):1] - 5. Daffalla, S.B., Mukhtar, H. and Shaharun, M.S. (2012) Effect of Organic and Inorganic Acid Pretreatment on Structural Properties of Rice Husk and Adsorption Mechanism of Phenol. International Journal of Chemical and Environmental Engineering, 3, 192-200. [Citation Time(s):1]
- 6. Dursun, G., Ciçek, H. and Dursun, A.Y. (2005) Adsorption of Phenol from Aqueous Solution by Using Carbonised Beet Pulp. Journal of Hazardous Materials, 125, 175-182.
https://doi.org/10.1016/j.jhazmat.2005.05.023 [Citation Time(s):1] - 7. Tseng, R.-L., Wu, K.-T., Wu, F.-C. and Juang, R.-S. (2000) Kinetic Studies on the Adsorption of Phenol, 4-Chlorophenol, and 2,4-Dichlorophenol from Water Using Activated Carbons. Journal of Environmental Management, 91, 2208-2214.
https://doi.org/10.1016/j.jenvman.2010.05.018 [Citation Time(s):1] - 8. TRIFI, I.M. (2013) Thèse de Doctorat en cotutelle Docteur en Chimie Etude de l’élimination du chrome (VI) par adsorption sur l’alumine activée et par dialyse ionique croisée. Créteil, Université Paris-est. [Citation Time(s):1]
- 9. Pramanpol, N. and Nitayapat, N. (2006) Adsorption of Reactive Dye by Eggshell and Its Membrane. Kasetsart Journal: Natural Science, 40, 192-197. [Citation Time(s):1]
- 10. Chowdhury, S., Chakraborty, S. and Das Saha, P. (2012) Removal of Crystal Violet from Aqueous Solution by Adsorption onto Eggshells: Equilibrium, Kinetics, Thermodynamics and Artificial Neural Network Modeling. Waste and Biomass Valorization, 4, 655-664.
https://doi.org/10.1007/s12649-012-9139-1 [Citation Time(s):1] - 11. Tsai, W.-T., Hsien, K.-J., Hsu, H.-C., Lin, C.-M., Lin, K.-Y. and Chiu, C.-H. (2008) Utilization of Ground Eggshell Waste as an Adsorbent for the Removal of Dyes from Aqueous Solution. Bioresource Technology, 99, 1623-1629.
https://doi.org/10.1016/j.biortech.2007.04.010 [Citation Time(s):1] - 12. Arami, M., Yousefi Limaee, N. and Mahmoodi, N.M. (2006) Investigation on the Adsorption Capability of Egg Shell Membrane towards Model Textile Dyes. Chemosphere, 65, 1999-2008.
https://doi.org/10.1016/j.chemosphere.2006.06.074 [Citation Time(s):2] - 13. Ahmad, R., Kumar, R. and Haseeb, S. (2012) Adsorption of Cu2+ from Aqueous Solution onto Iron Oxide Coated Eggshell Powder: Evaluation of Equilibrium, Isotherms, Kinetics, and Regeneration Capacity. Arabian Journal of Chemistry, 5, 353-359.
https://doi.org/10.1016/j.arabjc.2010.09.003 [Citation Time(s):1] - 14. Yeddou, N. and Bensmaili, A. (2007) Equilibrium and Kinetic Modelling of Iron Adsorption by Eggshells in a Batch System: Effect of Temperature. Desalination, 206, 127-134.
https://doi.org/10.1016/j.desal.2006.04.052 [Citation Time(s):1] - 15. Köse, T.E. and Kivanç, B. (2011) Adsorption of Phosphate from Aqueous Solutions Using Calcined Waste Eggshell. Chemical Engineering Journal, 178, 34-39.
https://doi.org/10.1016/j.cej.2011.09.129 [Citation Time(s):3] - 16. Boukhlifi, F., Chraibi, S. and Alami, M. (2013) Evaluation of the the Adsorption Kinetics and Equilibrium for the Potential Removal of Phenol Using a New Biosorbent. Journal of Environment and Earth Science, 3, 181-190. [Citation Time(s):5]
- 17. Xie, M., Chen, W., Xu, Z., Zheng, S. and Zhu, D. (2014) Adsorption of Sulfonamides to Demineralized Pine Wood Biochars Prepared under Different Thermochemical Conditions. Environmental Pollution, 186, 187-194.
https://doi.org/10.1016/j.envpol.2013.11.022 [Citation Time(s):1] - 18. Yang, K., Yang, J., Jiang, Y., Wu, W. and Lin, D. (2016) Correlations and Adsorption Mechanisms of Aromatic Compounds on a High Heat Temperature Treated Bamboo Biochar. Environmental Pollution, 210, 57-64.
https://doi.org/10.1016/j.envpol.2015.12.004 [Citation Time(s):1] - 19. Park, H.J., Jeong, S.W., Yang, J.K., Kim, B.G. and Lee, S.M. (2007) Removal of Heavy Metals Using Waste Eggshell. Journal of Environmental Sciences, 19, 1436-1441.
https://doi.org/10.1016/S1001-0742(07)60234-4 [Citation Time(s):3] - 20. Witoon, T. (2011) Characterization of Calcium Oxide Derived from Waste Eggshell and Its Application as CO2 Sorbent. Ceramics International, 37, 3291-3298.
https://doi.org/10.1016/j.ceramint.2011.05.125 [Citation Time(s):2] - 21. Hernández-Montoya, V., Ramírez-Montoya, L.A., Bonilla-Petriciolet, A. and Montes-Morán, M.A. (2012) Optimizing the Removal of Fluoride from Water Using New Carbons Obtained by Modification of Nut Shell with a Calcium Solution from Egg Shell. Biochemical Engineering Journal, 62, 1-7.
https://doi.org/10.1016/j.bej.2011.12.011 [Citation Time(s):1] - 22. Liu, F., Zhao, J., Wang, S. and Xing, B. (2016) Adsorption of Sulfonamides on Reduced Graphene Oxides as Affected by pH and Dissolved Organic Matter. Environmental Pollution, 210, 85-93.
https://doi.org/10.1016/j.envpol.2015.11.053 [Citation Time(s):1] - 23. Belaid, K.D. and Kacha, S. (2011) &EACUTE;tude Cinétique Et Thermodynamique De L’Adsorption D’Un Colorant Basique Sur La Sciure De Bois. Revue des Sciences de l’Eau, 24, 131-144.
https://doi.org/10.7202/1006107ar [Citation Time(s):2] - 24. Arami, M., Limaee, N. and Mahmoodi, N. (2008) Evaluation of the Adsorption Kinetics and Equilibrium for the Potential Removal of Acid Dyes Using a Biosorbent. Chemical Engineering Journal, 139, 2-10.
https://doi.org/10.1016/j.cej.2007.07.060 [Citation Time(s):1] - 25. Giles, H.A. and Smith, C.H. (1974) A general Treatment and Classification of the Solute Adsorption Isotherm. Journal of Colloid and Interface Science, 47, 755-765.
https://doi.org/10.1016/0021-9797(74)90252-5 [Citation Time(s):1] - 26. Giles, C.H., MacEwan, T.H., Nakhwa, S.N. and Smith, D. (1960) A System of Classification of Solution Adorption Isotherms and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. Journal of the Chemical Society, 3, 3973-3993.
https://doi.org/10.1039/jr9600003973 [Citation Time(s):1] - 27. Dubus, L. (1997) La rétention du phosphore dans les sols: Principes d’étude, modélisation, mécanismes et compartiments du sol impliquésle. Doc. Sci. Tech. III3, no. édition Centre ORSTOM de Nouméa. [Citation Time(s):2]
- 28. Salim, B. (2012) Etude de la dégradation photocatalytique et de l’adsorption du Phénol sur TiO2 P25. Influence de la présence des métaux lourds et des ultrasons. Université Mentouri Constantine Faculté des Sciences de l’Ingénieur. [Citation Time(s):2]
For further details log on website :
http://file.scirp.org/Html/2-1060130_72533.htm






No comments:
Post a Comment